Team:Tyngsboro MA Tigers/Results
From 2012hs.igem.org
(→Circuit 2) |
(→Circuit 2) |
||
| Line 415: | Line 415: | ||
== <B>Circuit 2</B> == | == <B>Circuit 2</B> == | ||
| - | If Circuit 2 was built, the following procedure would be employed for <b>BBa_K352003</b> and <b>BBa_J45199</b> to build the second plasmid in this system. The first plasmid, <b>BBa_352015</b> will have to be built from individual parts since it is not available in the Parts Registry | + | If Circuit 2 was built, the following procedure would be employed for <b>BBa_K352003</b> and <b>BBa_J45199</b> to build the second plasmid in this system. The first plasmid, <b>BBa_352015</b> will have to be built from individual parts since it is not available in the Parts Registry. |
<b>3A Assembly Protocol</b> | <b>3A Assembly Protocol</b> | ||
Refer to http://partsregistry.org/Help:3A_Assembly_Kit | Refer to http://partsregistry.org/Help:3A_Assembly_Kit | ||
Revision as of 17:07, 15 June 2012

Contents |
Results
Abstract
In the United States alone, carbon monoxide is responsible for over 2,100 deaths every year, with our only current defense being the warning sounds of carbon monoxide alarms. Carbon monoxide (CO) cannot be seen or smelled, so our project idea was to develop a biological alternative to the typical carbon monoxide detector to produce a warning smell, rather than a sound. Our circuit consists of the CO transcriptional activator CooA. In the presence of a CO inducer and an isoamyl alcohol precursor, the characteristic smell of bananas will be produced. Our team has developed a proof of concept circuit using the banana odor generating device as a reporter and the Tetracycline repressor as a constitutive generator to activate the transcription of ATF1 in an indole-free chassis. This circuit will replace CO gas with doxycycline as an inducer and isoamyl alcohol as a precursor to generate the banana smell.
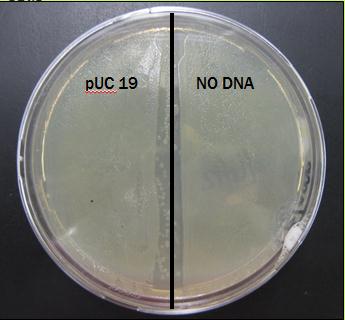
pUC 19 Transformation
The goal: Perform a transformation using pUC 19, a plasmid with high transformation efficiency, to test the quality of our protocol before using it for our experimental transformation.
The hypothesis: If the pUC 19 transformed successfully into E. coli, then only those cells will grow on ampicillin plates because they contain an ampicillin resistance chain.
The results: The plate was covered in bacteria on both pUC 19 side and NO DNA side. Results for this preliminary test are presently inconclusive.
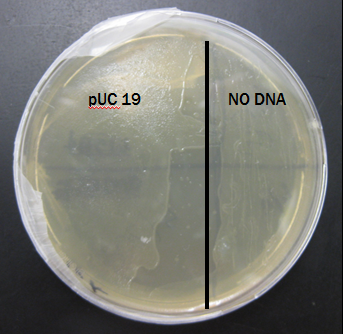
Expected Results
This plate shows the expected results for the transformation:
The pUC 19 side has bacteria growth and the No DNA side has no growth.
pUC 19 Transformation
Materials
Streaking from agar stabs:
70% ethanol
paper towels
marker/Sharpie
Agar Stab: - BBa_J45999 (kit)
Agar Stab: NEB10 beta strain (kit)
(6) inoculating loops (kit)
(2) LB agar plates – Amp/Kan (kit)
(1) SOB/LB agar plate (kit)
Growing up cell cultures:
(2) 14ml culture tubes (kit)
10ml of LB broth - Amp/Kan (kit)
(2) inoculating loops (kit)
Agar plate: Part A – BBa_J45999
rotator/shaker
Transformation(s)
ice
container for ice
timer
NEB10 cells (transformation 1)
Transformation 1 cells (transformation 2)
(3) 2.0ml microcentrifuge tubes (kit)
CaCl2 solution (kit)
(3) inoculating loops (kit)
SOC media (kit)
(3) LB agar plates (kit)
glass beads (kit)
waterbath
thermometer
Protocol
Part One
1. Label 2 small eppendorf tubes with “Puc 19” and “No DNA.”
2. Pipet 200 ul of CaCl2 transformation solution into each eppendorf tube and then place them on ice.
3. Use a sterile inoculating loop to gently scrape up one entire patch of the indole free cells and then swirl the cells into the “Puc 19” tube of cold CaCl2. Repeat the step for the “No DNA” tube of cold CaCl2.
4. Gently flick and invert the eppendorf tube to re-suspend the cells.
5. Keep competent cells on ice while you prepare the DNA for transformation.
Part Two
1. Retrieve the Puc 19 sample.
2. Add 2 ul of pUC 19 into the tube labeled “pUC 19”. Flick to mix the tube and return it to ice. Do not add pUC 19 to the tube labeled “No DNA."
3. Let the DNA and the cells sit on ice for 5 minutes.
4. While the cells are incubating, take a marker and label the bottom of the LB and ampicillin agar petri dish. Draw a line through the middle and label one side “Puc 19” and the other side “No DNA”, then add the date.
5. Heat shock both samples by placing the tubes at 42°C for 90 seconds exactly.
6. At the end of the 90 seconds, move the tubes to a rack at room temperature.
7. Add .5 ml of room temperature LB to the tubes. Close the caps and invert the tubes to mix the contents.
8. Using a sterile spreader, spread 250 ul of the “Puc 19” solution onto the designated half of the petri dish.
9. Using a new sterile spreader, spread 250 ul of the “No DNA” solution onto the designated half of the petri dish.
10. Cover the plate and set it aside for a minute, then turn the plate over.
11. Incubate the petri dish upside down overnight at 37°C.
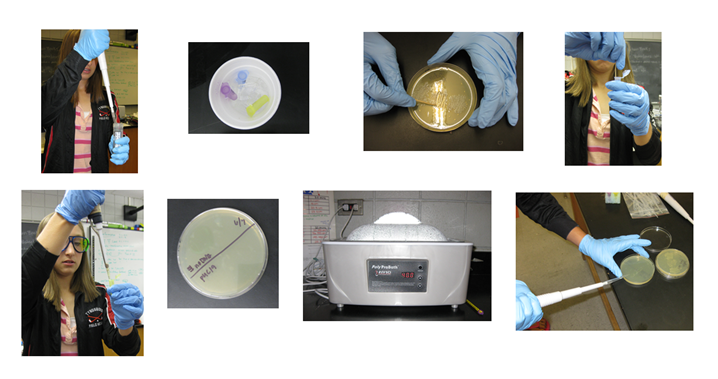
Pictures from performing different steps of the pUC19 transformation
Step 1: Pipeting CaCl2 (transformation solution) into both test tubes.
Step 2: Keeping the samples on ice.
Step 3: Scraping a patch of indole free cells.
Step 4: Adding the cells to the samples.
Step 5: Re-suspending the cells in the sample.
Step 6: Labeling the agar plate with a pUC 19 and No DNA side.
Step 7: Heat shocking the samples for 90 seconds at 42 degrees C.
Step 8: Spreading the samples onto the plates.
Results From First Trial
The plate was covered in bacteria on both sides after just 24 hours of incubation.
Results From Second Trial
The plate was also covered in bacteria on both sides after just 24 hours of incubation.
If colonies form, we will use the following procedure to determine the transformation efficiency of the plasmid. Determining Transformation Efficiency
Circuit 1
Once the transformation efficiency has been determined for pUC19, the transformation protocol will be repeated for BBa_J45200 (on ampicillin backbone). The transformants of this process will be used in a second transformation for BBa_K145201 (on kanamycin backbone). Colonies that grow on kan/amp agar plates will be selected for testing our proof of concept.
Materials
Streaking from agar stabs:
70% ethanol
paper towels
marker/Sharpie
Agar Stab: - BBa_J45999 (kit)
Agar Stab: NEB10 beta strain (kit)
(6) inoculating loops (kit)
(2) LB agar plates – Amp/Kan (kit)
(1) SOB/LB agar plate (kit)
Growing up cell cultures:
(2) 14ml culture tubes (kit)
10ml of LB broth - Amp/Kan (kit)
(2) inoculating loops (kit)
Agar plate: BBa_J45999
rotator/shaker
Preparing NEB 10-beta cells (2x)
Agar plate: NEB 10-beta strain (see previous step)
(1) SOB/LB Agar plate (kit)
(2) inoculating loops (kit)
distilled water (kit)
(1) 0.6ml tube (kit)
glass beads (kit)
Re-suspending DNA
Part BBa_J45200
Pipet
Distilled Water
Transformation(s)
ice
container for ice
timer
NEB10 cells (transformation 1)
Transformation 1 cells (transformation 2)
(6) 2.0ml microcentrifuge tubes (kit)
CaCl2 solution (kit)
(6) inoculating loops (kit)
Part BBa_J45200
SOC media (kit)
(6) LB agar plates (kit)
glass beads (kit)
water bath
thermometer
Procedure
Streaking from agar stabs
1. Clean lab bench by wiping down with 70% ethanol and paper towels.
2. Label an agar plate (ampicillin) with Indole Free or BBa_J45999.
3. Label an L.B agar plate with NEB 10-beta.
4. Use an inoculating loop to transfer some cells from the agar stab to the plate. There is a hole in each agar stab from where it was inoculated. Dip an inoculating loop into the stab at the same location, and streak the bacteria onto the agar plate in a zig-zag pattern. Using a fresh inoculating loop, streak onto the agar plate again creating a new zig-zag pattern that overlaps the first. This will help ensure that you will have single colonies to pick from. Streak gently, and try not to puncture the agar.
5. Repeat step 4 for the NEB 10-beta strain.
6. Place the agar plates into the incubator upside down at 37°C for 14-16 hours or room temperature for 24-30 hours.
7. After incubating they can be stored in a fridge at 4°C until a culture can be grown.
Growing up cell cultures
1. Clean the lab bench by wiping down with 70% ethanol and paper towels.
2. Remove the agar plates from the incubator or fridge.
3. Label one 14 mL culture tube for part J45999. Add 5 mL of LB broth (amp) to each culture tube.
4. Use an inoculating loop to pick a single colonly from each agar plate and inoculate the LB broth. Press lightly on the snap cap of the 14 mL tube, the capes should be a bit loose to allow for air flow.
5. Incubate for 16 hours at 37°C in a rotator or shaker.
6. After incubation, the cell culture should be cloudy. You can now firmly press down on the snap caps to seal the tubes and store the cell culture at 4°C until you’re ready to move onto the next step.
Preparing NEB 10-beta cells (competent cells)
1. Remove the agar plate for NEB 10-beta from the incubator of 4°C fridge.
2. Label one 0.6ml tube with "NEB 10-beta" and add 100ul of distilled water to it.
3. Use an inoculating loop, or pipette tip, to pick a single colony from the NEB 10-beta agar plate and inoculate the distilled water.
4. Pipette up and down to re-suspend the colony into the distilled water, and transfer to the fresh SOB agar plate.
5. Spread the cells across the plate using either an inoculating loop, or by pouring 3-6 glass beads into the plate, covering, and shaking from side to side.
6. Place the SOB agar plate into the incubator with the agar side facing up, lid facing down (see picture below). Incubate the plate at 37°C for 14-16 hours. Alternately, incubate at room temperature for 24-30 hours.
7. Once your agar plate has grown up you can store it in your fridge (4°C) until you're ready to make competent cells.
Re-suspending
1. Use a pipette to re-suspend part J45200 with 10 microliters of distilled water. Aspirate up and down a few times to ensure the DNA has been fully re-suspended.
Transformation
1. Obtain a slant of Bba-J45999 indole free chassis.
2. Label one closed micro test tube +J45200 and another -J45200. Place them in the foam tube rack.
3. Open the tubes and, using a sterile transfer pipet, transfer 250 microliters of transformation solution (CaCl2).
4. Place the tubes on ice.
5. Use a sterile loop to pick up a single colony of bacteria from the starter plate. Pick up the +J45200 tube and immerse the loop in the transformation solution at the bottom of the tube. Spin the loop between your index finger and thumb until the entire colony is dispersed in the transformation solution (with no floating chunks). Place the tubes back in the tube rack in the ice. Using a new sterile loop, repeat for the -J45200 tube.
6. Immerse a new sterile loop into the stock tube containing the re-suspended J45200 plasmid. Withdraw a loopful.
There should be a film of plasmid solution across the ring. Mix the loopful into the cell suspension of the +J45200 tube. Close the tube and return it to the rack on ice. Also close the -J45200 tube. Do not add plasmid DNA to the -J45200.
7. Incubate the tubes on ice for 10 minutes. Make sure to push the tube all the way down in the rack so the bottoms of the tubes stick out and make contact with the ice.
8. While the tubes are sitting on ice, label your four LB nutrient agar plates on the bottom sides as follows:
LB/amp +
LB/amp –
LB +
LB -
9. Using the foam rack as a holder, transfer both the +J45200 and -J45200 tubes into the water bath set at 42 degrees Celsius, for exactly 50 seconds. Make sure to push the tubes all the way down in the rack so the bottoms of the tubes stick out and make contact with the warm water.
10. Directly after the 50 seconds, place both tubes back on ice (0° C). Incubate the tubes on ice for 2 minutes.
11. Remove the rack containing the tubes from the ice and place on the bench top. Open a tube and, using a new sterile pipet, add 250 microliters of LB nutrient broth to the tube and reclose it. Repeat with a new sterile pipet for the other tube. Incubate the tubes for 10 minutes at room temperature.
12. Tap the closed tubes with your finger to mix. Pipet 100 microliters of the transformation suspension (+J45200) onto the LB/amp + plate using a new sterile pipet. Pipet 100 microliters of the control suspension (-J45200) onto the LB/amp – plate, using a new sterile pipet.
13. Spread the suspensions evenly around the surface of the agar using the flat surface of a sterile loop. Use a new sterile loop for each plate.
14. Stack up your plates and tape them together. Put the stack upside down in the incubator at 37°C until the next day.
Transformation of Second Plasmid
A. Make competent cells
1. Remove transformation plate LB/amp + from the incubator or fridge.
2. Label one 0.6ml tube with "NEB 10-beta 2" and add 100ul of distilled water to it.
3. Use an inoculating loop, or pipette tip, to pick a single colony from the LB/amp + plate and inoculate the distilled water.
4. Pipette up and down to re-suspend the colony into the distilled water, and transfer to the fresh SOB agar plate.
5. Spread the cells across the plate using either an inoculating loop, or by pouring 3-6 glass beads into the plate, covering, and shaking from side to side.
6. Place the SOB agar plate into the incubator with the agar side facing up, lid facing down (see picture below). Incubate the plate at 37°C for 14-16 hours. Alternately, incubate at room temperature for 24-30 hours.
7. Once your agar plate has grown up you can store it in your fridge (4°C) until you're ready to make competent cells.
B. Re-suspending
1. Use a pipette to re-suspend part K145201 with 10 microliters of distilled water. Aspirate up and down a few times to ensure the DNA has been fully re-suspended.
C. Transformation
1. Remove plate from the incubator. This plate is the LB/amp + sample used to make competent cells.
2. Label one tube +K145201/J45200 and one tube +J45200.
3. Open the tubes and, using a sterile transfer pipet, transfer 250 microliters of transformation solution (CaCl2).
4. Place the tubes on ice.
5. Use a sterile loop to pick up a single colony of bacteria from the LB/amp + competent cells plate. Pick up the +K145201 and immerse the loop in the transformation solution at the bottom of the tube. Spin the loop between your index finger and thumb until the entire colony is dispersed in the transformation solution (with no floating chunks). Place the tubes back in the tube rack in the ice. Using a new sterile loop, repeat for the +J45200 tube.
6. Immerse a new sterile loop into the stock tube containing the re-suspending K145201 plasmid. Withdraw a loopful. There should be a film of plasmid solution across the ring. Mix the loopful into the cell suspension of the +K145201/J45200 tube. Close the tube and return it to the rack on ice. Also close the +J45200 tube. Do not add plasmid DNA to the -J45200.
7. Incubate the tubes on ice for 10 minutes. Make sure to push the tube all the way down in the rack so the bottoms of the tubes stick out and make contact with the ice.
8. While the tubes are sitting on ice, label your LB nutrient agar plates on the bottom sides as follows:
LB/amp/kan +
LB/amp +
LB +
LB –
9. Using the foam rack as a holder, transfer the three tubes into the water bath set at 42° C, for exactly 50 seconds. Make sure to push the tubes all the way down in the rack so the bottoms of the tubes stick out and make contact with the warm water.
10. Directly after the 50 seconds, place all three tubes back on ice (0° C). Incubate the tubes on ice for 2 minutes.
11. Remove the rack containing the tubes from the ice and place on the bench top. Open a tube and, using a new sterile pipet, add 250 microliters of LB nutrient broth to the tube and reclose it. Repeat with a new sterile pipet for the other two tubes. Incubate the tubes for 10 minutes at room temperature.
12. Tap the closed tubes with your finger to mix.
13. Pipet 100 microliters of the transformation suspension +K145201/J45200 onto the LB/amp/kan + using a new sterile pipet. Repeat this step, using new sterile pipets, for the LB+ and LB- plates.
14. Pipet 100 microliters of the transformation suspension +J45200 onto the LB/amp + using a new sterile pipet. Repeat this step, using new sterile pipets, for the LB+ and LB- plates.
15. Spread the suspensions evenly around the surface of the agar using the flat surface of a sterile loop. Use a new sterile loop for each plate.
16. Stack up your plates and tape them together. Put the stack upside down in the incubator at 37°C until the next day.
Circuit 2
If Circuit 2 was built, the following procedure would be employed for BBa_K352003 and BBa_J45199 to build the second plasmid in this system. The first plasmid, BBa_352015 will have to be built from individual parts since it is not available in the Parts Registry.
3A Assembly Protocol
Refer to http://partsregistry.org/Help:3A_Assembly_Kit
 "
"