Team:BVCAPS Research KS/Project
From 2012hs.igem.org
Gtwehmeyer (Talk | contribs) |
Gtwehmeyer (Talk | contribs) |
||
| Line 7: | Line 7: | ||
This system is the combination of the MIT and University of Texas teams' projects from years past. We are making a combined genetic circuit with the smell of the bacteria (banana or mint) depending on the time of day (presence of light). The light receptor protein, obtained from photosynthetic algae, turns the banana smell on in the absence of light, and the mint smell on in the presence of light. In the agar of the plates we use for the final system we would need to include the substrates isoamyl alcohol and salicyclic acid. | This system is the combination of the MIT and University of Texas teams' projects from years past. We are making a combined genetic circuit with the smell of the bacteria (banana or mint) depending on the time of day (presence of light). The light receptor protein, obtained from photosynthetic algae, turns the banana smell on in the absence of light, and the mint smell on in the presence of light. In the agar of the plates we use for the final system we would need to include the substrates isoamyl alcohol and salicyclic acid. | ||
| + | |||
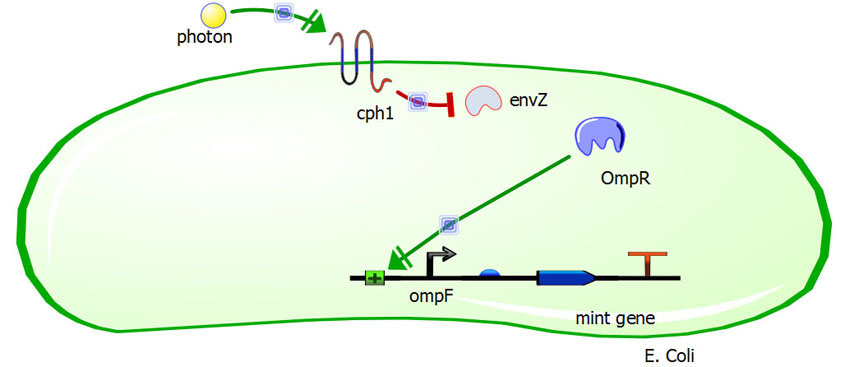
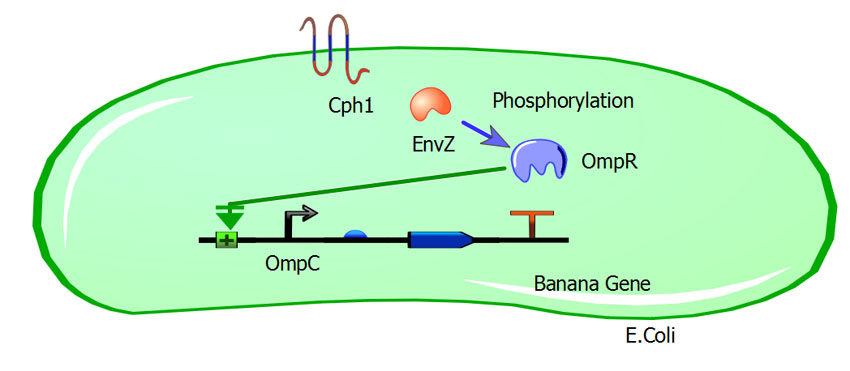
| + | In the dark, the EnvZ protein in the membrane protein complex cph8 (BBa_I15010) is more highly activated, which phosphorylates a greater amount of the transcription factor OmpR, which naturally occurs in E. coli. Large amounts of OmpR-P, in turn, will suppress the OmpF promoter (BBa_K116500), which controls the mint odor generator (BBa_J45004). OmpR-P will also activate the OmpC promoter (BBa_R0082), which controls the Banana odor generator (BBa_J45199). Thus banana smell at night and no mint odor generated. The opposite is intended to occur in the daylight. When a photon hits the receptor of cph8, EnvZ activity is reduced, causing less OmpR phosphorylation, causing activation of the OmpF promoter, yielding a mint odor, while the OmpC promoter is no longer activated, ceasing the production of the banana odor. | ||
| + | |||
| + | We will be co-transforming a single strain of E.coli with our two systems contained within two separate plamids. The images below have been presented as two separate systems for clarity sake, but both plasmids will exist in a single organism. | ||
Visual of our system in presence of light | Visual of our system in presence of light | ||
| Line 18: | Line 22: | ||
==Parts== | ==Parts== | ||
| - | Banana odor enzyme generator (BBa_J45199)- contains RBS, coding region, | + | Banana odor enzyme generator (BBa_J45199)- contains RBS, coding region, two terminators, location well 10B plate 1, resistant to amp/kan. It was isolated from yeast used in alcohol fermentation which would give it its unique smell. |
Mint odor generator (BBa_J45004)- contains coding region, location is well 23B plate 3 and Amp resistant. This gene was isolated from petunia and snapdragon plants. It breaks down salicyclic acid into methyl sali to give it a mint smell. | Mint odor generator (BBa_J45004)- contains coding region, location is well 23B plate 3 and Amp resistant. This gene was isolated from petunia and snapdragon plants. It breaks down salicyclic acid into methyl sali to give it a mint smell. | ||
| Line 24: | Line 28: | ||
Light sensor protein or Cph8 (BBa_I15010)- contains coding region, location is well 20D plate 3 and is Kanamycin resistant. This part was taken from a UT Tech previous iGEM project. If a photon hits the light sensor Cph1 it will then inhibit EnvZ. | Light sensor protein or Cph8 (BBa_I15010)- contains coding region, location is well 20D plate 3 and is Kanamycin resistant. This part was taken from a UT Tech previous iGEM project. If a photon hits the light sensor Cph1 it will then inhibit EnvZ. | ||
| - | + | OmpC promoter (BBa_R0082)is used as promoter for banana gene sequence so it is activated in high amounts of OmpRp at nighttime. | |
| - | + | ||
| - | + | ||
| - | OmpF | + | OmpF promoter (BBa_K116500) is used as promoter for mint gene sequence so it is activated in the day when low amounts of OmpRp are present. |
==Human Practices== | ==Human Practices== | ||
Revision as of 21:13, 16 June 2012
Overview
Our project, in essence, designs the perfect alarm clock. The presence of a strong wintergreen smell is used to help the user wake up, heightening their senses. At night, when no light is present, a banana odor produced will assist in calming down the user, helping them go to bed. This all occurs in the absence of electricity through pure biological systems.
This system is the combination of the MIT and University of Texas teams' projects from years past. We are making a combined genetic circuit with the smell of the bacteria (banana or mint) depending on the time of day (presence of light). The light receptor protein, obtained from photosynthetic algae, turns the banana smell on in the absence of light, and the mint smell on in the presence of light. In the agar of the plates we use for the final system we would need to include the substrates isoamyl alcohol and salicyclic acid.
In the dark, the EnvZ protein in the membrane protein complex cph8 (BBa_I15010) is more highly activated, which phosphorylates a greater amount of the transcription factor OmpR, which naturally occurs in E. coli. Large amounts of OmpR-P, in turn, will suppress the OmpF promoter (BBa_K116500), which controls the mint odor generator (BBa_J45004). OmpR-P will also activate the OmpC promoter (BBa_R0082), which controls the Banana odor generator (BBa_J45199). Thus banana smell at night and no mint odor generated. The opposite is intended to occur in the daylight. When a photon hits the receptor of cph8, EnvZ activity is reduced, causing less OmpR phosphorylation, causing activation of the OmpF promoter, yielding a mint odor, while the OmpC promoter is no longer activated, ceasing the production of the banana odor.
We will be co-transforming a single strain of E.coli with our two systems contained within two separate plamids. The images below have been presented as two separate systems for clarity sake, but both plasmids will exist in a single organism.
Visual of our system in presence of light
Visual of our system in the dark
Parts
Banana odor enzyme generator (BBa_J45199)- contains RBS, coding region, two terminators, location well 10B plate 1, resistant to amp/kan. It was isolated from yeast used in alcohol fermentation which would give it its unique smell.
Mint odor generator (BBa_J45004)- contains coding region, location is well 23B plate 3 and Amp resistant. This gene was isolated from petunia and snapdragon plants. It breaks down salicyclic acid into methyl sali to give it a mint smell.
Light sensor protein or Cph8 (BBa_I15010)- contains coding region, location is well 20D plate 3 and is Kanamycin resistant. This part was taken from a UT Tech previous iGEM project. If a photon hits the light sensor Cph1 it will then inhibit EnvZ.
OmpC promoter (BBa_R0082)is used as promoter for banana gene sequence so it is activated in high amounts of OmpRp at nighttime.
OmpF promoter (BBa_K116500) is used as promoter for mint gene sequence so it is activated in the day when low amounts of OmpRp are present.
Human Practices
Outreach
In early May team members Brandon Whitcomb and Tim Schaefer taught a lesson on genetic engineering and its societal impacts to three seperate second grade classes at Cottonwood Pint elementary school in Overland Park, Kansas.
The BVCAPS Research Kansas team took on the first annual CAPS Bioengineering Summer Camp and taught them the value of exposure and knowledge of bioengineering in a world fast heading in that direction. We presented our project to these students and presented the steps necessary to achieve a successful bacterial transformation.
In response to our work with these students we have verbal commitments from a good selection of the 23 students saying they would be interested in participating in iGem in the future.
On the Friday leading up to the freeze we took in one urban child who we found was fascinated by Biology and Bioengineering. He was quick to understand the practical uses of a lot of our lab equipment i.e. vortex machine, micro-pipets, centrifuge machine, and electrophoresis machine. At just 8 years old he took on the tasks we presented to him with flying colors and limited team member help.
 "
"