Team:Lethbridge Canada/Notebook
From 2012hs.igem.org
(→Transformation of TAT-E1010_pGEM (x2) and J23100-Mlc_pGEM (x1) into DH5α) |
(→June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)) |
||
| (15 intermediate revisions not shown) | |||
| Line 692: | Line 692: | ||
| - | [[Image:May 23rd PCR tubes E1010 fusion.JPG|left| | + | [[Image:May 23rd PCR tubes E1010 fusion.JPG|left|552px]][[Image:May23.JPG|right|245px]] <br> |
| Line 1,090: | Line 1,090: | ||
*Incubated on ice for 30mins <br> | *Incubated on ice for 30mins <br> | ||
*Incubated 45secs in water bath @ 42˚C <br> | *Incubated 45secs in water bath @ 42˚C <br> | ||
| - | * | + | *Immediately incubated on ice for 5mins <br> |
*Added 400µL of SOC media; resuspended <br> | *Added 400µL of SOC media; resuspended <br> | ||
*Put in shaker for 1hr @ 37 ˚C <br> | *Put in shaker for 1hr @ 37 ˚C <br> | ||
*200µL cultured onto plate(4 plates, Amp) <br> | *200µL cultured onto plate(4 plates, Amp) <br> | ||
*Incubate @ 37 ˚C for approximately 16hrs <br> | *Incubate @ 37 ˚C for approximately 16hrs <br> | ||
| - | |||
| - | |||
==='''June 6, 2012: Gel of J23100-Mlc_pGEM Restriction (June 4)'''=== | ==='''June 6, 2012: Gel of J23100-Mlc_pGEM Restriction (June 4)'''=== | ||
| Line 1,146: | Line 1,144: | ||
*Put 1.5mL of transformed cells in a microcentrifuge tube; centrifuge and repeat until cells from entire tube are in the microcentrifuge tube. <br> | *Put 1.5mL of transformed cells in a microcentrifuge tube; centrifuge and repeat until cells from entire tube are in the microcentrifuge tube. <br> | ||
*Resuspend pelleted bacterial cells in 250uL Buffer P1 <br> | *Resuspend pelleted bacterial cells in 250uL Buffer P1 <br> | ||
| - | *Add | + | *Add 250µL Buffer P2, mix by inversion <br> |
| - | *Add | + | *Add 350µL Buffer N3, mix by inversion <br> |
*Centrifuge for 10 mins @ 13000 rpm <br> | *Centrifuge for 10 mins @ 13000 rpm <br> | ||
*Put supernatant into the QIAprep spin column <br> | *Put supernatant into the QIAprep spin column <br> | ||
| - | *Centrifuge for 60s | + | *Centrifuge for 60s @ 14000 rpm, discard flow through <br> |
| - | *Add | + | *Add 750µL buffer PE and centrifuge for 60s @ 14000 rpm <br> |
*Centrifuge again to get rid of remaining buffer <br> | *Centrifuge again to get rid of remaining buffer <br> | ||
| - | *Add | + | *Add 50µL of Buffer EB to spin columns, let stand for 1 min, centrifuge for 1 min at 14000 rpm <br> |
*3 samples were made and ran on a gel, all samples appeared to be the expected size | *3 samples were made and ran on a gel, all samples appeared to be the expected size | ||
| - | |||
| - | |||
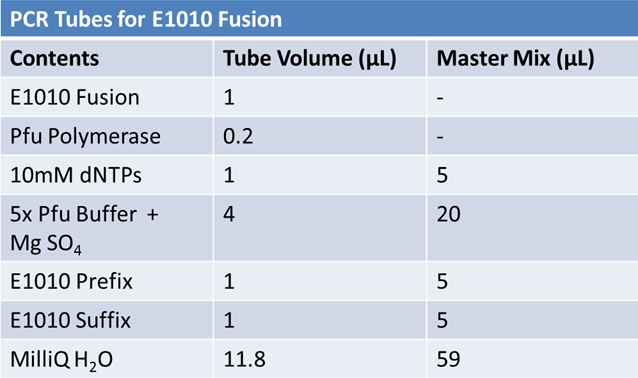
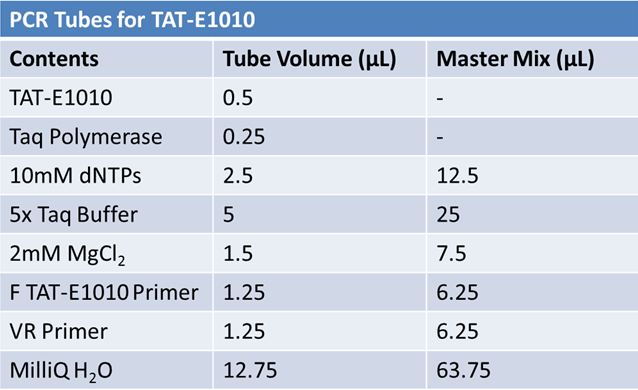
===PCR of E1010 Fusion (to confirm part) and TAT-E1010 (for insertion into pGEM)'''=== | ===PCR of E1010 Fusion (to confirm part) and TAT-E1010 (for insertion into pGEM)'''=== | ||
| Line 1,202: | Line 1,198: | ||
| - | [[Image: | + | [[Image:June 6th PCR tubes TAT-E1010.JPG|left|638px]] |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 1,269: | Line 1,235: | ||
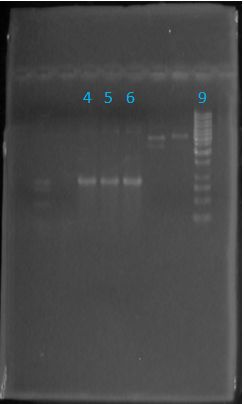
| + | [[Image:June 6th PCR Gel lanes TAT-E1010.JPG|left|px]][[Image:June6tat.JPG|center|244px]] | ||
| Line 1,275: | Line 1,242: | ||
*Add 24.25 µL of the Master Mix into 4 microcentrifuge tubes | *Add 24.25 µL of the Master Mix into 4 microcentrifuge tubes | ||
*Add TAT-E1010 and Taq polymerase | *Add TAT-E1010 and Taq polymerase | ||
| + | |||
| Line 1,325: | Line 1,293: | ||
*All TAT-E1010 samples worked | *All TAT-E1010 samples worked | ||
* Sample from lane 5 was ligated with pGEM | * Sample from lane 5 was ligated with pGEM | ||
| - | |||
| - | |||
===June 7th 2012: Transformation of TAT-E1010_pGEM (x2) and J23100-mlc_pGEM into DH5ɑ=== | ===June 7th 2012: Transformation of TAT-E1010_pGEM (x2) and J23100-mlc_pGEM into DH5ɑ=== | ||
| - | *Added 2.0 µL of DNA to 20 | + | *Added 2.0 µL of DNA to 20 µL of DH5ɑ. |
*Incubated on ice for 30 minutes. | *Incubated on ice for 30 minutes. | ||
| Line 1,347: | Line 1,313: | ||
*Incubated for approximately 16 hours at 37⁰C. | *Incubated for approximately 16 hours at 37⁰C. | ||
| - | |||
| - | |||
===June 10th 2012: Restriction of J23100-mlc with E and X and J06702 with S and P and Ligation of J23100-mlc with J06702 (pSB1A2) === | ===June 10th 2012: Restriction of J23100-mlc with E and X and J06702 with S and P and Ligation of J23100-mlc with J06702 (pSB1A2) === | ||
| Line 1,361: | Line 1,325: | ||
<br> | <br> | ||
| + | |||
| + | |||
| + | |||
| Line 1,421: | Line 1,388: | ||
<br> | <br> | ||
| + | |||
| + | |||
| + | |||
| Line 1,477: | Line 1,447: | ||
[[Image:June 10th ligation 2.JPG|left|px]] | [[Image:June 10th ligation 2.JPG|left|px]] | ||
| - | |||
| Line 1,575: | Line 1,544: | ||
*Incubated for approximately 16 hours at 37 degrees Celsius. | *Incubated for approximately 16 hours at 37 degrees Celsius. | ||
| - | |||
| - | |||
===June 12th, 2012: Picking Cells and Making M9 Media=== | ===June 12th, 2012: Picking Cells and Making M9 Media=== | ||
| Line 1,628: | Line 1,595: | ||
*Shake for 1 hour at 37oC for approx. 16 hours | *Shake for 1 hour at 37oC for approx. 16 hours | ||
| - | ===Insulin Secretion | + | ===Insulin Secretion Proof of Principle'''=== |
Picked 4 cultures from -80 freezer stocks: <br> | Picked 4 cultures from -80 freezer stocks: <br> | ||
| Line 1,702: | Line 1,669: | ||
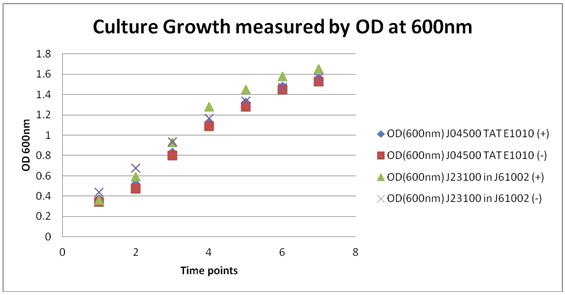
| - | Culture Samples were spun down and at 8000rpm for 2 minutes and supernatant was taken and ran through a fluorescent spectrophotometer for excitation of RFP at 586nm and expected emission at 607nm. | + | Culture Samples were spun down and at 8000rpm for 2 minutes and supernatant was taken and ran through a fluorescent spectrophotometer for excitation of RFP at 586nm and expected emission at 607nm. |
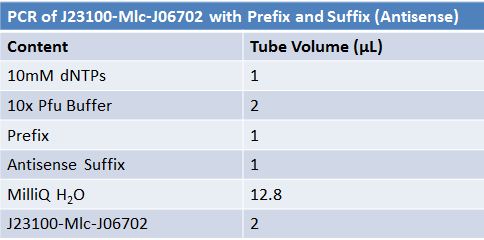
| + | ==='''June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)'''=== | ||
| - | |||
[[Image:June 16 PCR J23100-Mlc-J06702.JPG|left|px]] | [[Image:June 16 PCR J23100-Mlc-J06702.JPG|left|px]] | ||
| - | + | (See [https://2012hs.igem.org/Team:Lethbridge_Canada/Results "Results"].) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 03:53, 17 June 2012

| Home | The Team | The Project | Results | Human Practices | Notebook | Safety |
|---|
NotebookMarchBrainstorming Project Ideas
The chosen project
Project outlinePossible things to consider:
AprilSub-groupsWe divided the team into sub-groups. Each group assumed responsibility for a different aspect of the project. Wiki and Mascot Design This group is responsible to obtaining and uploading all necessary information to the wiki. This includes team pictures and biographies, experiments and results, and the projects of the other sub-groups. This will be a priority over mascot design.Leader: Erin Kelly
This group must document the safety protocols that the Lethbridge team practices in the lab.Leader: Marissa Guzzi
This group is responsible for letting the public know what our team is and what we are doing. This can be done creatively. The point is to raise awareness for iGEM, synthetic biology, and diabetes.Leader: Chris Isaac WHMIS tests and Safety TrainingWe are required to have safety training in order to work in the lab, and so we all wrote our WHMIS tests and did our Hazards Assesment and lab orientation! MayMay 8, 2012: Tansformation of Parts from Kit Plate and Growing Cells from Glycerol StocksTransformation of parts from kit plate:
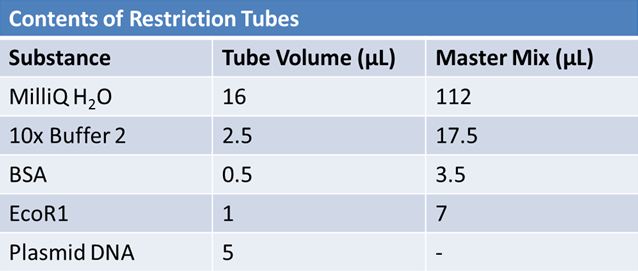
May 9, 2012: Miniprep and Restriction of PartsOvernight cultures from May 8th were miniprepped and restricted in order to determine sizes.
Master mix was prepared by mixing the ingredients together in order, as shown above. 20µL worth of master mix was combined with 5µL of plasmid DNA:
Restrictions were incubated at 37˚C for 30mins then stored at -20˚C.
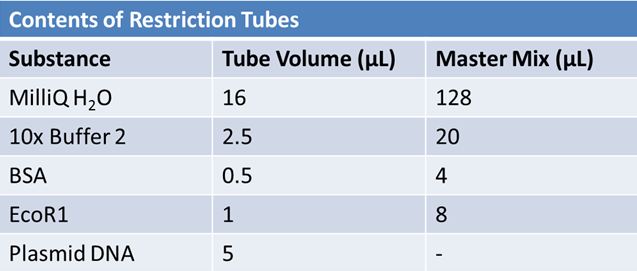
May 10, 2012: Miniprep and Restriction of PartsOvernight cultures from May 9th were miniprepped and restricted in order to determine sizes
Restrictions were incubated at 37˚C for 30min then stored in -20˚C.
May 11th: Electrophoresis of restricted parts from May 10th
Purpose: confirm existence of the following parts:
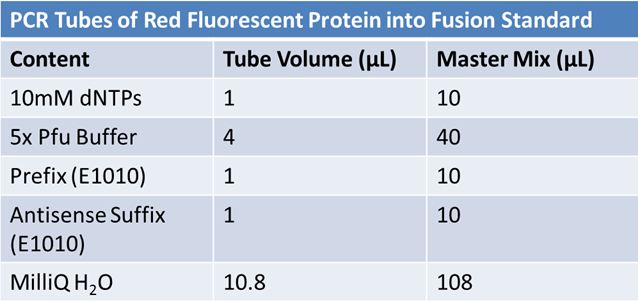
PCR of RFP (E1010) into fusion standard (adding extra bp to get into fusion standard) Add 17.8 µL to 8 tubes with: May 12th: Assembly of promoter & RBS (J04500) with signal sequence (K331009) and RPF (E1010 fusion) with double terminator (B0015)Method: Restriction, gel extraction, and ligation Restriction:
We made a 1% TAE agarose gel and ran restrictions for 1 hour @ 120V We then gel extracted the DNA Ligation:
May 13th Transformation of ligated products: J04500 + K331009 and E1010 (fusion) + B0015Transformation: 2µL of each ligation mix was added to 20µL DH5ɑ cells
May 14th: Picking Cells from May 13th transformation
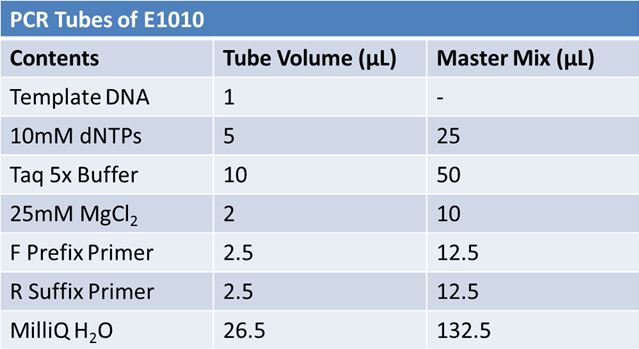
May 19th: PCR of E1010 and J23100 (Mlc primers)E1010:
Ran 1.5% agarose TAE gel at 90V for 45 mins
May 20: Re-do May 19 PCR of E1010 (fusion) and J23100-mlcAlterations to PCR:
PCR cycle:
May 21st: PCR of J23100 and E1010 repeat again!
For both J20100 and E1010:
PCR protocol:
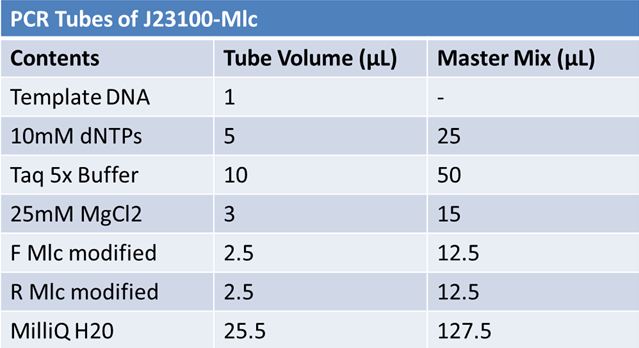
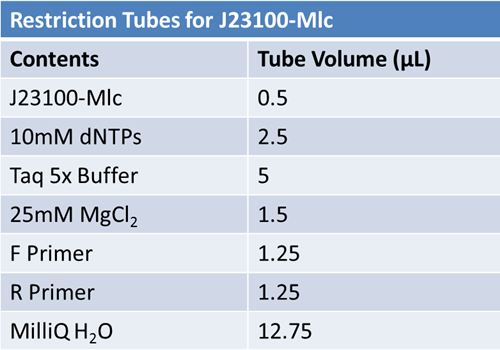
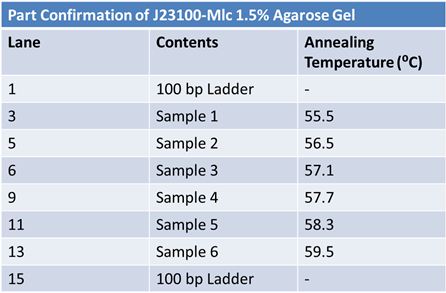
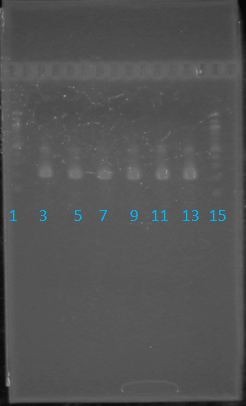
May 22, 2012: PCR of J23100-MlcPCR Parameters: Initial denaturation 95˚C 3mins 30 cycles of: *denaturation 95˚C 30s *annealing (various) 30s *elongation 72˚C 1min
1.5% agarose gel Ran at 120v for 1 hr
May 23, 2012: Ligation of PCR products into pGEM Vector
2) Prefix-(fusion)-E1010-suffix (Black tubes) (sample 1 from May 23rd PCR) Contents:
May 23, 2012 Transformation of J23100-Mlc and E1010 fusionFor each ligation mix:
-0.45 agarose M -30 mL TAE (1x)
May 24, 2012: Transformation of PCR Products in pGEM Vectors in DH5ɑSample 3 from PCR product ligation For each ligation mix:
May 24 picking colonies of J20100-mlc and E1010 in DH5ɑFor each sample:
May 25, 2012: QIA Spin Miniprep of J23100-mlc (A: colony 1), J23100-mlc (B: colony 2)
May 26 gel of Miniprep samples
May 29, 2012: Transformation of E1010 fusion in pGEM in DH5ɑFor each ligation mix:
May 30 QIA spin MiniprepDNA:
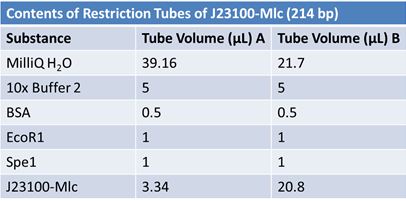
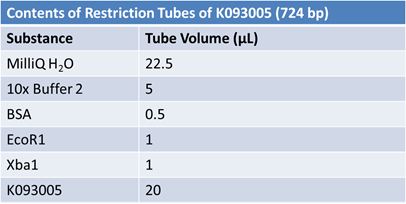
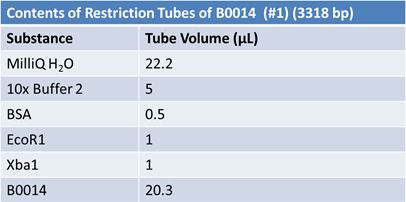
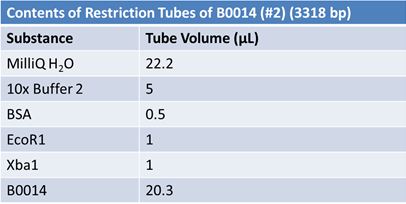
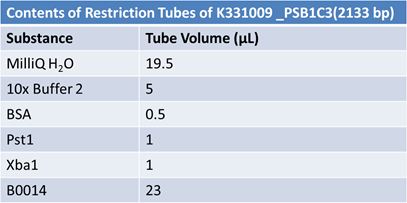
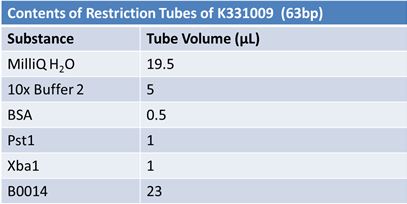
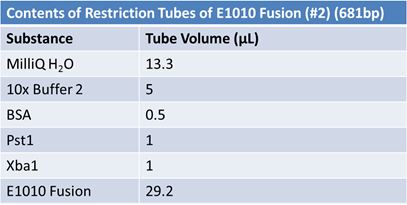
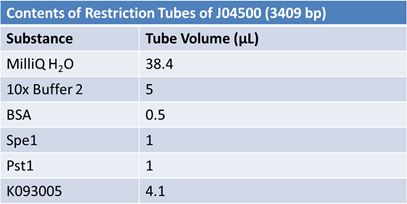
JuneJune 1, 2012: Restriction of J23100-Mlc, K093005, B0014 (samples 1 and 2), K331009_PSB1C3, K331009, E1010 Fusion (samples 1 and 2), J04500
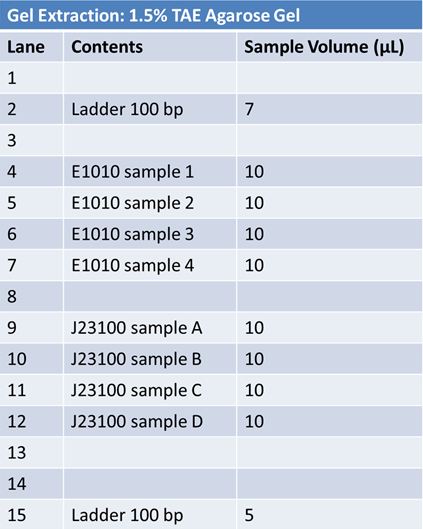
Gel Extraction
June 2, 2012: Miniprep of June 1 Cultures
June 3, 2012: Confirmaiton of Parts
What worked:
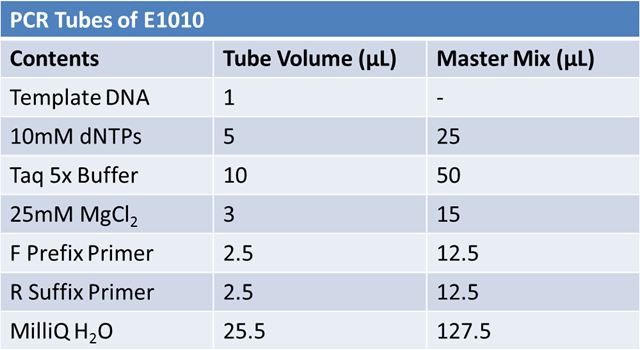
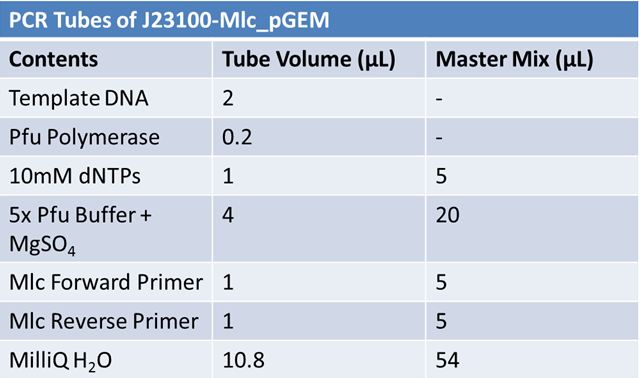
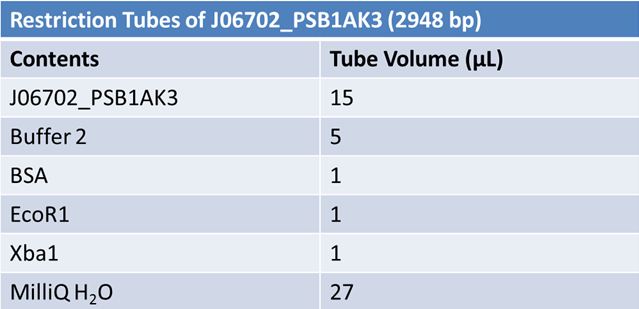
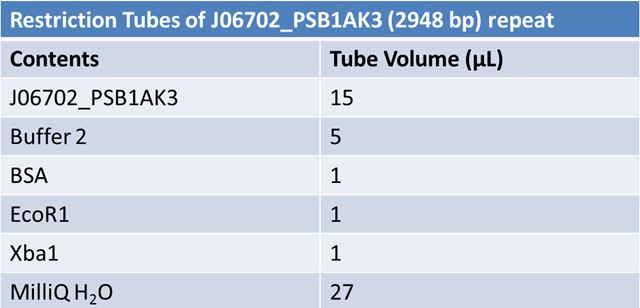
June 4, 2012: PCR to Confirm J23100-Mlc in pGEM
Transformation of TAT-E1010_pGEM (x2) and J23100-Mlc_pGEM (x1) into DH5αFor each ligation mix:
June 5, 2012: Transformation of J06702 (mCherry RFP) into DH5α
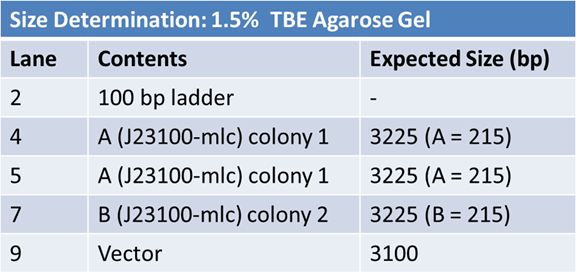
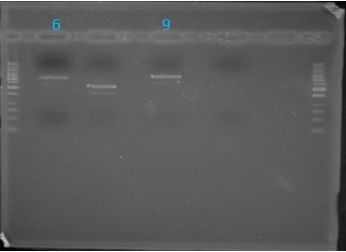
June 6, 2012: Gel of J23100-Mlc_pGEM Restriction (June 4)
Miniprep of J06702
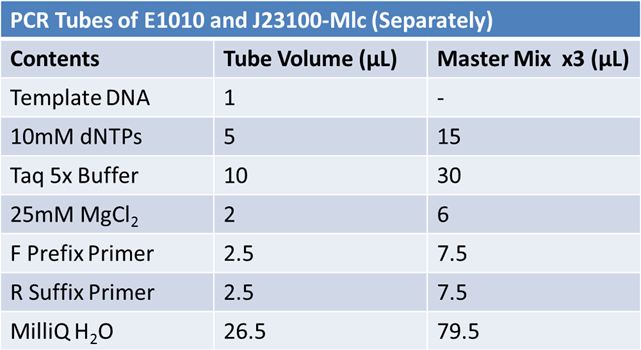
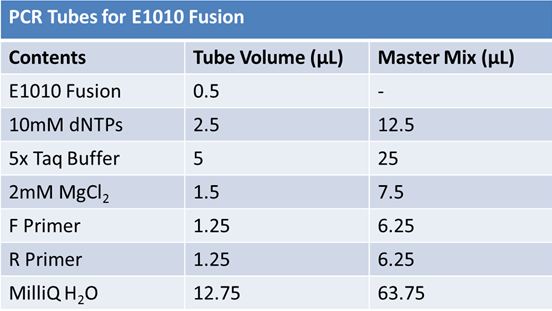
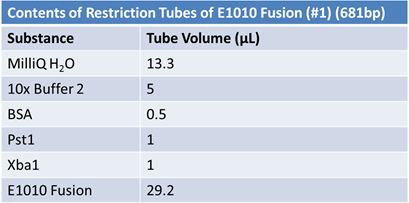
PCR of E1010 Fusion (to confirm part) and TAT-E1010 (for insertion into pGEM)
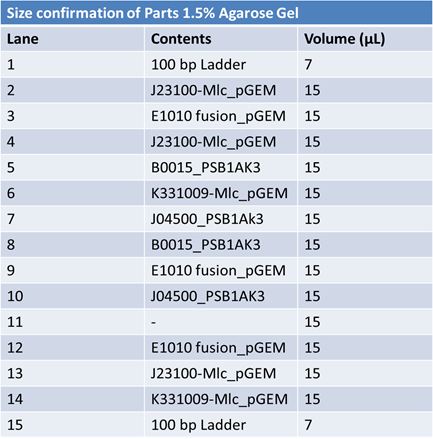
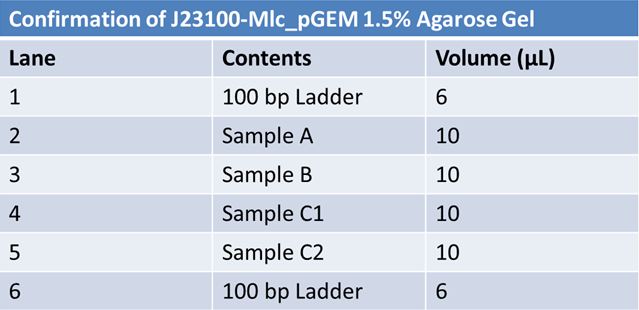
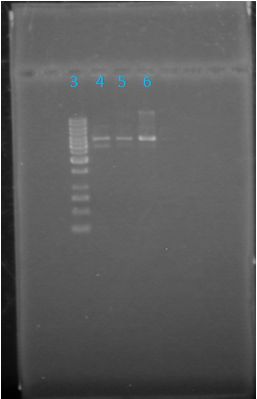
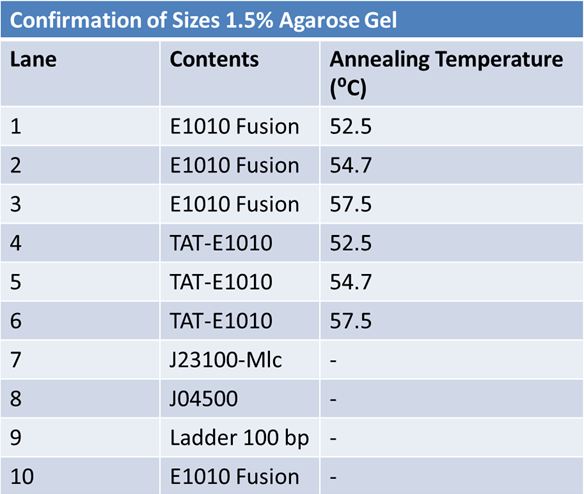
Size Confirmation Gel of E1010 fusion, TAT-E1010, J23100-Mlc, J04500
June 7th 2012: Transformation of TAT-E1010_pGEM (x2) and J23100-mlc_pGEM into DH5ɑ
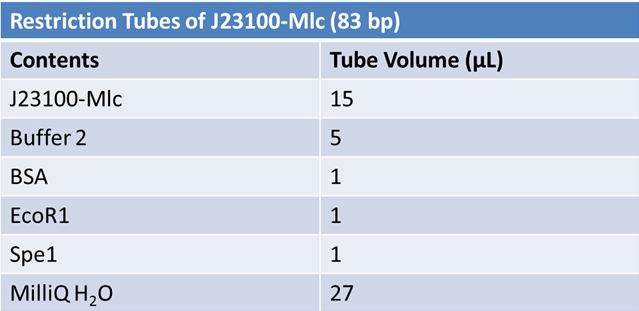
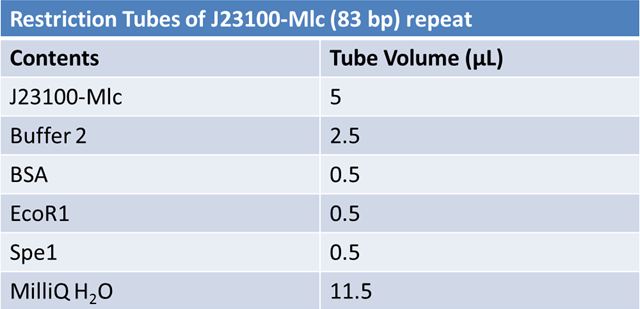
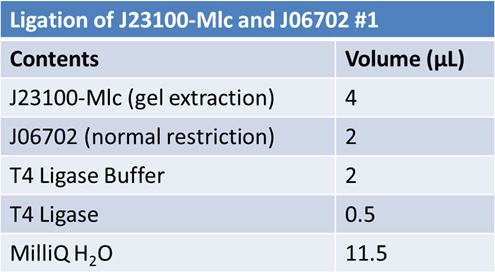
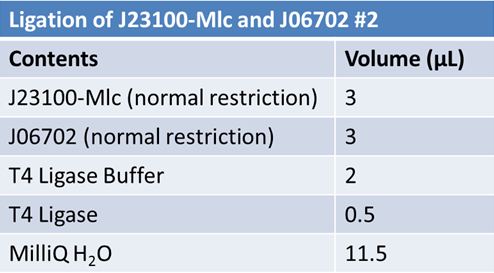
June 10th 2012: Restriction of J23100-mlc with E and X and J06702 with S and P and Ligation of J23100-mlc with J06702 (pSB1A2)Restriction:
June 11th 2012: Transformation of J23100-mlc-J06702 samples from June 10thFor each ligation mix:
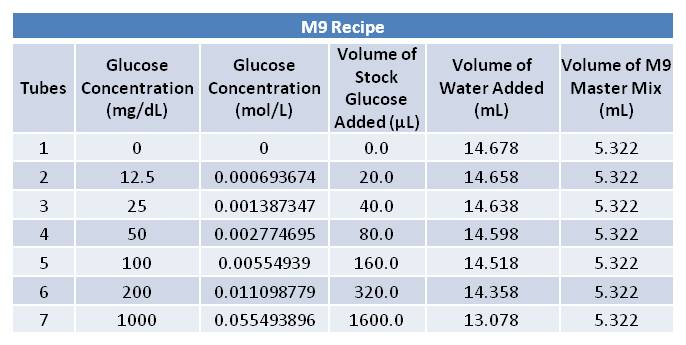
June 12th, 2012: Picking Cells and Making M9 Media
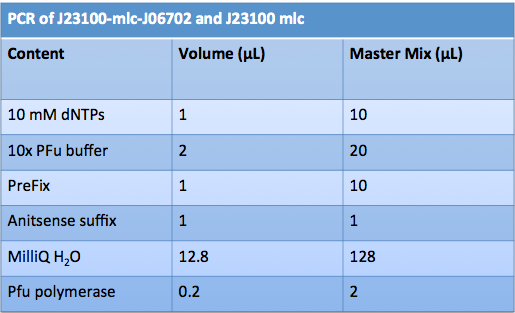
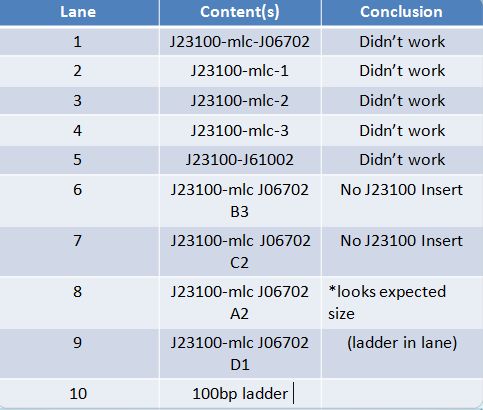
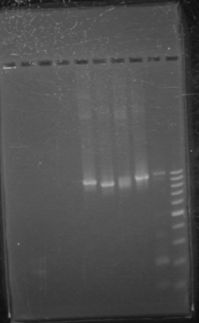
Aliquoted as two 10 mL samples of the same glucose concentration. Replacing a portion of the water added with various volumes of 0.693674 mol/L glucose solution as follows: June 14th, 2012 PCR of J23100-Mlc-J06702 and J23100-Mlc
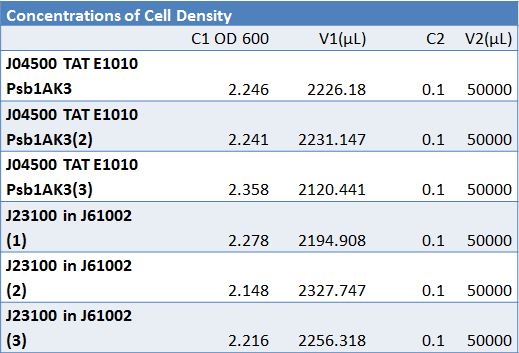
June 15, 2012:Transformation of Plasmid PSB1C3 with J23100-Mlc and K093005 and Insulin Secretion Proof of PrinclipleTransformation:
Insulin Secretion Proof of PrinciplePicked 4 cultures from -80 freezer stocks:
Cultures were grown over night
The re-suspended cells were added to 50 ml cultures and 50ul of Ampicillin was also added to media. Cultures were placed in the shaker and shaken at 37 ⁰C.
June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)(See "Results".) |
 "
"