Team:Lethbridge Canada/Notebook
From 2012hs.igem.org
(→May) |
(→June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)) |
||
| (214 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
</html> | </html> | ||
{| style="color:#000000;background-color:#b5cde2;" cellpadding="6" cellspacing="3" border="0" bordercolor="#00FFFF" width="65%" align="center" | {| style="color:#000000;background-color:#b5cde2;" cellpadding="6" cellspacing="3" border="0" bordercolor="#00FFFF" width="65%" align="center" | ||
| - | !align="center"|[[Image: | + | !align="center"|[[Image:Note pic 4.jpg|center|900px]] |
|} | |} | ||
| Line 30: | Line 30: | ||
='''Notebook'''= | ='''Notebook'''= | ||
==March== | ==March== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | <i> Brainstorming Project Ideas</i> | ||
| + | * <b>Pests</b> | ||
| + | **Bacterial Pest attractor (engineer bacteria to produce a substance, such as a pheromone or smell, that attracts and kills pests such as insects). | ||
| + | **Natural pesticides (engineer bacteria to produce a substance that repels or kills pests, such as those that harm crops, that can later be implemented into plants). <br> <br> | ||
| - | + | * <b>Water/Environment</b> | |
| - | Desalination of water (engineering bacteria to get rid of salt in salt water in order to make drinkable water from sea water) | + | **Desalination of water (engineering bacteria to get rid of salt in salt water in order to make drinkable water from sea water). |
| - | Getting rid of estrogen mimicking compounds in water (engineering bacteria to degrade them or sequester them) | + | **Getting rid of estrogen mimicking compounds in water (engineering bacteria to degrade them or sequester them) |
| - | Waste treatment (engineering bacteria that can be integrated into waste water treatment) | + | **Waste treatment (engineering bacteria that can be integrated into waste water treatment). |
| - | CFCs (engineer bacteria to produce metabolites that break down chlorofluorocarbons—compounds that contribute to the degradation of the ozone layer) <br> | + | **CFCs (engineer bacteria to produce metabolites that break down chlorofluorocarbons—compounds that contribute to the degradation of the ozone layer).<br><br> |
| + | * <b>Health </b> | ||
| + | **Stomach ulcers (creating a medication containing engineered bacteria to specifically target and kill Helicobacter pylori – the organism that causes stomach ulcers). | ||
| + | **Diabetes (engineered bacteria as Islet cells to produce insulin). | ||
| + | **Allergies/Immune system (engineer bacteria to produce antihistamines or alter epitopes). | ||
| + | **Bone density (engineer bacteria to produce and secrete calcium and other compounds to help heal broken bones or to prevent osteoporosis). <br><br> | ||
| - | + | * <b>Process Improvement </b> | |
| - | + | **Oil fractionation catalyst (engineering bacteria to improve the separation of crude oil into valuable fraction and waste fraction). | |
| - | + | **Nitrate fixation (engineer bacteria to improve nitrogen fixation so not as much fertilizer is needed). <br><br> | |
| - | + | ||
| - | + | ||
| - | + | * <b>Kill Switches </b> | |
| - | + | **Engineering bacteria to undergo induced or programmed cell death in order to control the organism). <br><br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===The chosen project=== | ===The chosen project=== | ||
| - | After dividing in to a few groups based on which project was preferred, each group made a presentation on the proposed project. In the end, we decided to go with the glucose sensing project. | + | *After dividing in to a few groups based on which project was preferred, each group made a presentation on the proposed project. In the end, we decided to go with the '''glucose sensing project'''.<br><br> |
===Project outline=== | ===Project outline=== | ||
| - | + | <i>Possible things to consider:</i> | |
| - | + | * <b>Glucose and insulin </b> | |
| - | + | **Detection of blood glucose levels (glucose-specific promoter?) | |
| - | + | **Insulin production release (measure/regulation of production?) <br> <br> | |
| - | + | * <b>Immune response </b> | |
| - | + | **Possibly use "bactoblood" strain from Berkeley 2007 <br><br> | |
| - | + | * <b>Kill switch</b> | |
| - | + | ||
| - | + | * <b>Delivery system </b> | |
| - | + | **Intravenous | |
| - | + | **NASA Biocapsule <br> | |
==April== | ==April== | ||
| + | |||
| + | ===Sub-groups=== | ||
| + | |||
We divided the team into sub-groups. Each group assumed responsibility for a different aspect of the project. | We divided the team into sub-groups. Each group assumed responsibility for a different aspect of the project. | ||
| - | + | '''Wiki and Mascot Design'''<br> | |
| + | <Blockquote>''This group is responsible to obtaining and uploading all necessary information to the wiki. This includes team pictures and biographies, experiments and results, and the projects of the other sub-groups. This will be a priority over mascot design.'' | ||
| + | <Blockquote>Leader: Erin Kelly<br> | ||
| + | 1. Erin Kwan<br> | ||
| + | 2. Alycia Amatto<br> | ||
| + | 3. Cassandra Logue<br> | ||
| + | 4. Teddi Reynolds<br> | ||
| + | 5. Dax Law<br> | ||
| + | 6. Riley Martens<br> | ||
| + | 7. Elaine Bird<br> | ||
| + | 8. Brooke Heatherington <br> | ||
| + | 9. Corbin Chenger <br> | ||
| + | 10. Devan Carrier <br> | ||
| + | </Blockquote></Blockquote> | ||
| - | |||
| - | + | '''Biosafety'''<br> | |
| + | <Blockquote>''This group must document the safety protocols that the Lethbridge team practices in the lab.'' | ||
| + | <Blockquote>Leader: Marissa Guzzi<br> | ||
| + | 1. Branden Black<br> | ||
| + | 2. Iain Sander<br> | ||
| + | 3. Carissa Kirk<br> | ||
| + | 4. Brianna Carrels<br> | ||
| + | 5. Amanda<br> | ||
| + | 6. Alli Herauf<br> | ||
| + | </Blockquote></Blockquote> | ||
| - | |||
| - | ''' | + | '''Human Practices'''<br> |
| + | <Blockquote>''This group is responsible for letting the public know what our team is and what we are doing. This can be done creatively. The point is to raise awareness for iGEM, synthetic biology, and diabetes.'' | ||
| + | <Blockquote>Leader: Chris Isaac<br> | ||
| + | 1. Shammamah Hossain<br> | ||
| + | 2. Dawson Meyer<br> | ||
| + | 3. Yoyo Yao<br> | ||
| + | 4. Jared Sparkes<br> | ||
| + | 5. Wesley Mosimann<br> | ||
| + | 6. Trisha Bouma<br> | ||
| + | 7. Janelle Veenendaal<br> | ||
| + | 8. Orion Sehn<br> | ||
| + | </Blockquote></Blockquote> | ||
| - | + | ===WHMIS tests and Safety Training=== | |
| - | + | <Blockquote>We are required to have safety training in order to work in the lab, and so we all wrote our WHMIS tests and did our Hazards Assesment and lab orientation!</Blockquote> | |
| - | + | ==May== | |
| - | ''' | + | ==='''May 8, 2012: Tansformation of Parts from Kit Plate and Growing Cells from Glycerol Stocks'''=== |
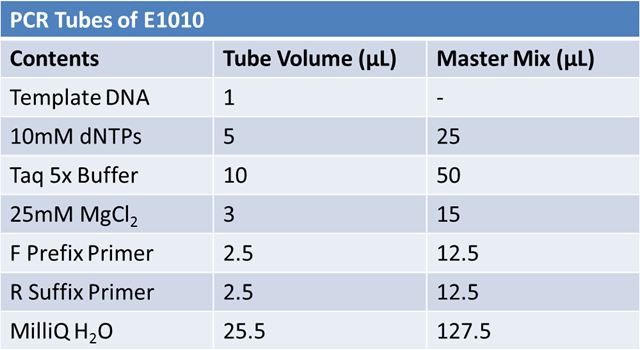
| - | + | Transformation of parts from kit plate: | |
| - | + | *B0034 in pSB1A2 <br> | |
| + | *K093005 in pSB1A2 <br> | ||
| + | *J23100 in J61002 (Amp) <br> | ||
| + | *E1010 in pSB2K3 <br> | ||
| + | |||
| + | |||
| + | 2µL of DNA was added to 20µL DH5ɑ cells | ||
| + | |||
| + | *Incubated on ice for 30 mins<br> | ||
| + | |||
| + | *Cells were then heat shocked for 45sec in water bath at 42 degrees Celsius<br> | ||
| + | |||
| + | *Immediately after, cells were incubated on ice for 5 mins<br> | ||
| - | + | *400µL SOC media was added and cells were resuspended<br> | |
| - | + | *Cells were then placed in shaker for 1h @ 37 degrees Celsius<br> | |
| - | + | *200µL of this culture was plated on AMP plates<br> | |
| - | + | *Plates were incubated at 37 degrees for appx. 16hrs.<br> | |
| - | |||
Overnight cultures were also made of: | Overnight cultures were also made of: | ||
| - | + | *pMA-T K33109 (HST-1)<br> | |
| + | *pSB1AK3 B0014<br> | ||
| + | |||
| + | ==='''May 9, 2012: Miniprep and Restriction of Parts'''=== | ||
| + | |||
| + | |||
| + | |||
| + | |||
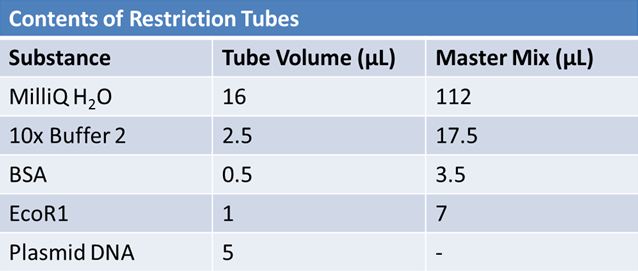
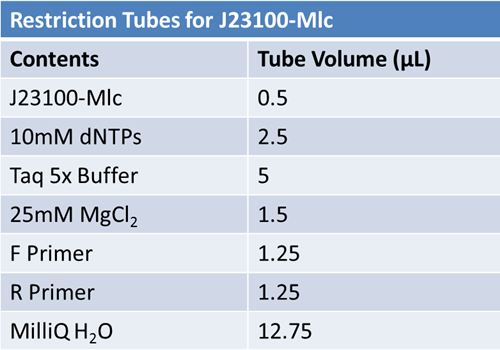
| + | Overnight cultures from May 8th were miniprepped and restricted in order to determine sizes. | ||
| + | |||
| + | |||
| + | [[Image:May 9th restriction tubes.JPG|left|638px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Master mix was prepared by mixing the ingredients together in order, as shown above. 20µL worth of master mix was combined with 5µL of plasmid DNA: | ||
| + | |||
| + | *pMA-T BBa_K331009 (Heat-stable toxin signal sequence)<br> | ||
| + | *pSB1AK3 BBa_B0014 (Double terminator)<br> | ||
| + | |||
| + | Restrictions were incubated at 37˚C for 30mins then stored at -20˚C. | ||
| + | |||
| + | |||
| + | Colonies were also picked. Overnight cultures were made of : | ||
| + | |||
| + | *E1010 pSB2K3<br> | ||
| + | *J23100 J61002 (Amp)<br> | ||
| + | *K93005 pSB1A2 <br> | ||
| + | *B0034 pSB1A2<br> | ||
| + | |||
| + | |||
| + | |||
| + | ==='''May 10, 2012: Miniprep and Restriction of Parts'''=== | ||
| + | |||
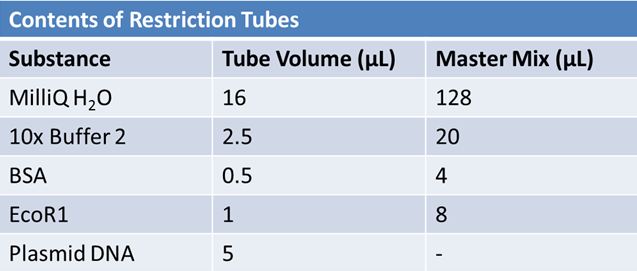
| + | Overnight cultures from May 9th were miniprepped and restricted in order to determine sizes | ||
| + | |||
| + | [[Image:May_10th_restriction_tubes.JPG |left|640px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Master mix was prepared by mixing the ingredients together in order, as shown above. 20µL worth of master mix was combined with 5µL of plasmid DNA (1-7). | Master mix was prepared by mixing the ingredients together in order, as shown above. 20µL worth of master mix was combined with 5µL of plasmid DNA (1-7). | ||
| - | + | *J23100 (J61002) <br> | |
| + | *J23100 (J61002)<br> | ||
| + | *B0034 (pSB1A3)<br> | ||
| + | *B0034 (pSB1A3)<br> | ||
| + | *E1010 (pSB2K3)<br> | ||
| + | *E1010 (pSB2k3)<br> | ||
| + | *K093008 (pSB1A2)<br> | ||
| - | - | + | Restrictions were incubated at 37˚C for 30min then stored in -20˚C. |
| - | |||
| - | |||
| - | + | ==='''May 11th: Electrophoresis of restricted parts from May 10th'''=== | |
| + | <br> | ||
| + | [[Image:May 11th PCR tubes.JPG|left|638px]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
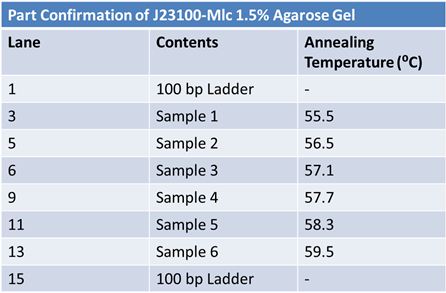
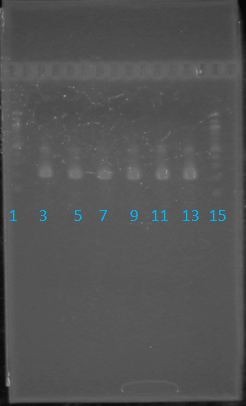
| - | + | Purpose: confirm existence of the following parts: | |
| - | + | *J23100 (J61002) <br> | |
| + | *B0034 (pSB1A3)<br> | ||
| + | *E1010 (pSB2K3)<br> | ||
| + | *K093008 (pSB1A2)<br> | ||
| - | Method: Restriction, gel extraction, | + | |
| + | *We also grew up J04500 in pSB1AK3 for assembly | ||
| + | |||
| + | |||
| + | |||
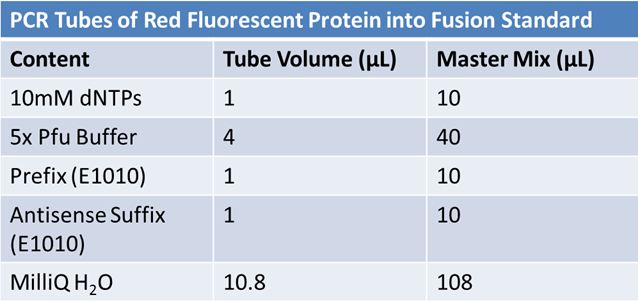
| + | PCR of RFP (E1010) into fusion standard | ||
| + | <Blockquote> | ||
| + | (adding extra bp to get into fusion standard) | ||
| + | |||
| + | Add 17.8 µL to 8 tubes with: <br> | ||
| + | *Pfu polymerase 0.2 µL <br> | ||
| + | *Template DNA 2 µL<br> | ||
| + | *Put tubes on gradient function program Pfu test with various annealing temperatures: | ||
| + | |||
| + | Annealing gradient (degrees celsius): <br> | ||
| + | |||
| + | *49.2 <br> | ||
| + | *51.6<br> | ||
| + | *53.0<br> | ||
| + | *54.5<br> | ||
| + | *55.9<br> | ||
| + | *57.3<br> | ||
| + | *58.8<br> | ||
| + | *61.2<br> | ||
| + | |||
| + | *only 54.5 worked <br> | ||
| + | </Blockquote> | ||
| + | |||
| + | ==='''May 12th: Assembly of promoter & RBS (J04500) with signal sequence (K331009) and RPF (E1010 fusion) with double terminator (B0015)'''=== | ||
| + | |||
| + | |||
| + | |||
| + | Method: Restriction, gel extraction, and ligation | ||
Restriction: | Restriction: | ||
| - | + | *J04500 <br> | |
| + | *K331009<br> | ||
| + | *E1010 (fusion)<br> | ||
| + | *B0015 <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | Gel extraction | + | Gel extraction: |
We made a 1% TAE agarose gel and ran restrictions for 1 hour @ 120V | We made a 1% TAE agarose gel and ran restrictions for 1 hour @ 120V | ||
| - | + | We then gel extracted the DNA | |
Ligation: | Ligation: | ||
| - | Ligated together J04500 & K331009 | + | *Ligated together J04500 & K331009 |
| - | Ligated together E1010 fusion & B0015 | + | *Ligated together E1010 fusion & B0015 |
| - | |||
| - | '''May 13th''' | + | ==='''May 13th Transformation of ligated products: J04500 + K331009 and E1010 (fusion) + B0015'''=== |
| - | |||
| - | + | Transformation: | |
| - | + | 2µL of each ligation mix was added to 20µL DH5ɑ cells | |
| - | + | *Incubated on ice for 30 mins<br> | |
| - | + | *Cells were then heat shocked for 45sec in water bath at 42 degrees Celsius<br> | |
| - | + | *Immediately after, cells were incubated on ice for 5 mins<br> | |
| - | + | *400µL SOC media was added and cells were resuspended<br> | |
| - | + | *Cells were then placed in the shaker for 1h @ 37 degrees<br> | |
| + | *200µL of this culture was plated on AMP plates<br> | ||
| - | + | *Plates were incubated at 37 degrees for appx. 16hrs.<br> | |
| - | Picking Cells from May 13th transformation | + | ==='''May 14th: Picking Cells from May 13th transformation'''=== |
| - | Cells grew on 2 plates, 2 colonies were picked | + | *Cells grew on 2 plates, 2 colonies were picked |
| - | Picked cells were put into 5ml LB media with kanamycin | + | *Picked cells were put into 5ml LB media with kanamycin |
| - | Cells did not grow | + | *Cells did not grow |
| - | |||
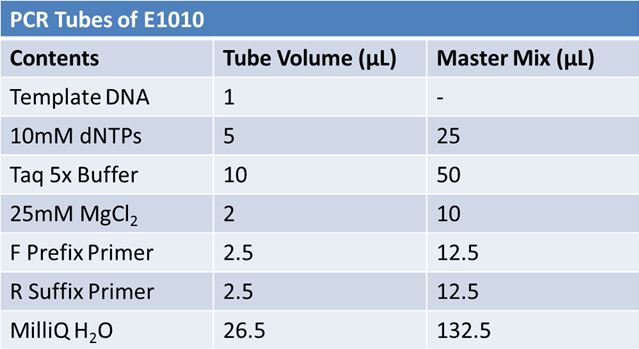
| - | + | ==='''May 19th: PCR of E1010 and J23100 (Mlc primers)'''=== | |
| - | |||
| - | E1010 | + | '''E1010:''' |
| - | [[Image: | + | [[Image:May 19th PCR E1010.JPG|left|640px]] |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | '''May 20: | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 49.5µL to 4 PCR tubes and add 0.5µL Taq polymerase and 1µL template DNA to each<br> | ||
| + | |||
| + | *E1010 (fusion) primer | ||
| + | *The annealing temp was 55.5˚C | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''J23100:''' | ||
| + | [[Image:May 19th PCR J23100-Mlc.JPG|left|639px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 49.5 µL of MM to 4 PCR tubes and add 0.5 µL Taq polymerase to each tube | ||
| + | |||
| + | *J23100-Mlc primer annealing temp- 58.0˚C | ||
| + | |||
| + | |||
| + | |||
| + | Ran 1.5% agarose TAE gel at 90V for 45 mins | ||
| + | |||
| + | [[Image:May 19th gel extraction.JPG|left|423px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *E1010 expected size: 700 bp<br> | ||
| + | *J23100 expected size: 215 bp<br> | ||
| + | |||
| + | *Did not work | ||
| + | |||
| + | ==='''May 20: Re-do May 19 PCR of E1010 (fusion) and J23100-mlc'''=== | ||
Alterations to PCR: | Alterations to PCR: | ||
| - | + | *2µL MgCl2 | |
PCR cycle: | PCR cycle: | ||
| - | + | *First Denaturation: 3 mins @ 94˚<br> | |
| + | *Denaturation: 15 sec @94˚<br> | ||
| + | *Annealing: 30sec E1010: 54.5˚ J23100: 55.0˚<br> | ||
| + | *Elongation: I min 30 sec @ 72˚<br> | ||
| + | *Final elongation: 7 mins @72˚<br> | ||
| - | |||
| - | + | [[Image:May 20th PCR E1010.JPG|left|639px]] | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | '''May 22, 2012: PCR of J23100-Mlc''' | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 49.5 µL of MM to 4 PCR tubes<br> | ||
| + | *Add 1 µL template DNA<br> | ||
| + | *Add 0.5 µL Taq DNA polymerase to each<br> | ||
| + | |||
| + | |||
| + | Didn’t work (again!) | ||
| + | |||
| + | |||
| + | |||
| + | ==='''May 21st: PCR of J23100 and E1010 repeat again!'''=== | ||
| + | |||
| + | [[Image:May 21st PCR E1010 and J23100.JPG|left|641px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
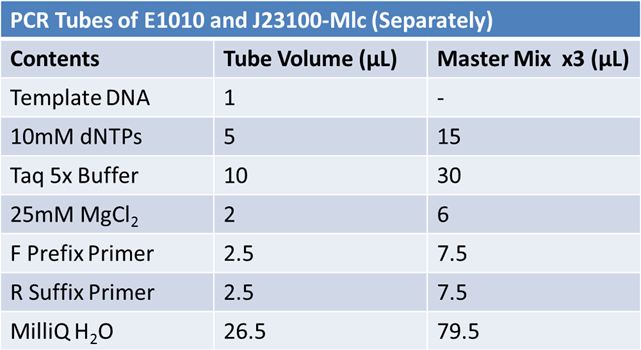
| + | For both J20100 and E1010: | ||
| + | |||
| + | *Put 45.9 µL of MM into x PCR tubes<br> | ||
| + | |||
| + | *Add 3 µL of template DNA | ||
| + | |||
| + | *PCR PFU test #4 | ||
| + | |||
| + | *Ran on 1.5% agarose gel at 120V for 1hour | ||
| + | |||
| + | PCR protocol: | ||
| + | |||
| + | *First Denaturation: 2 mins @ 95˚ | ||
| + | |||
| + | *Denaturation: 30 sec @95˚ | ||
| + | |||
| + | *Annealing: 30sec E1010: 54.5˚ J23100: 55.0˚ | ||
| + | |||
| + | *Elongation: I min 30 sec @ 72˚ | ||
| + | |||
| + | *First elongation: 7 mins @72˚ | ||
| + | |||
| + | *changed to 30 cycles | ||
| + | |||
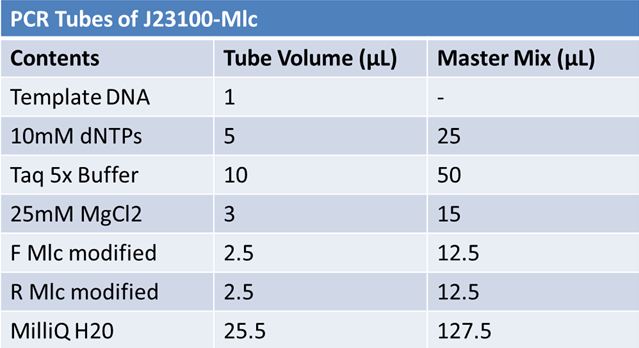
| + | ==='''May 22, 2012: PCR of J23100-Mlc'''=== | ||
PCR Parameters: | PCR Parameters: | ||
| Line 374: | Line 634: | ||
30 cycles of: | 30 cycles of: | ||
| - | + | *denaturation 95˚C 30s | |
| - | + | *annealing (various) 30s | |
| - | + | *elongation 72˚C 1min | |
| Line 385: | Line 645: | ||
PCR Samples: | PCR Samples: | ||
| - | [[Image: | + | [[Image:May 22nd restriction tubes.JPG|left|px]] |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 408: | Line 682: | ||
Ran at 120v for 1 hr | Ran at 120v for 1 hr | ||
| - | [[Image: | + | [[Image:May 22nd confirmation gel.JPG|left|px]] [[Image:May_22nd_PCR_gel.JPG|center|246px]] |
| - | |||
| - | |||
| - | |||
| - | |||
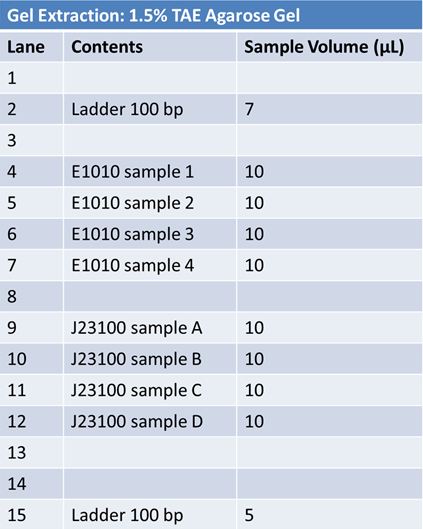
| - | + | ==='''May 23, 2012: Ligation of PCR products into pGEM Vector'''=== | |
| - | |||
| - | + | [[Image:May 23rd PCR tubes E1010 fusion.JPG|left|552px]][[Image:May23.JPG|right|245px]] <br> | |
| - | |||
| - | |||
| - | -Ligate at room temp for 1h 15 mins | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | 1) prefix –J23100-mlc-spe cut site (Red tubes) (sample 3 from May 22nd PCR) | ||
| + | |||
| + | 2) Prefix-(fusion)-E1010-suffix (Black tubes) (sample 1 from May 23rd PCR) | ||
| + | |||
| + | Contents: | ||
| + | |||
| + | *2x Rapid Ligation Buffer 5µL <br> | ||
| + | *pGEM-Tor pGem-T Easy Vector 1µL <br> | ||
| + | *PCR Product 3 µL <br> | ||
| + | *T4 DNA Ligase 1µL <br> | ||
| + | *MiliQ H2O 1 µL <br> | ||
| + | *Ligate at room temp for 1h 15 mins <br> | ||
*One of each sample ligated overnight @ 4˚c | *One of each sample ligated overnight @ 4˚c | ||
| Line 435: | Line 740: | ||
*2 of each ligated at room temperature for approx. 1hr | *2 of each ligated at room temperature for approx. 1hr | ||
| + | ==='''May 23, 2012 Transformation of J23100-Mlc and E1010 fusion'''=== | ||
| - | |||
For each ligation mix: | For each ligation mix: | ||
| - | + | *Added 2µL of ligation mix ( E1010 pcr + pGEM; J23100- mlc + pGEM) to 20.0µL of DH5α) <br> | |
| + | *Incubated on ice for 30mins <br> | ||
| + | *45secs in water bath @ 42˚C <br> | ||
| + | *immediately incubated on ice for 5mins <br> | ||
| + | *Added 400µL of SOC media; resuspended <br> | ||
| + | *Put in shaker for 1hr @ 37 ˚C <br> | ||
| + | *200µL cultured onto plate(4 plates, Amp) <br> | ||
| + | *Incubate @ 37 ˚C for approximately 16hrs <br> | ||
| - | |||
| - | - | + | Make: 1.5% agarose gel |
| + | |||
| + | -0.45 agarose M | ||
| - | - | + | -30 mL TAE (1x) |
| - | + | <br> | |
| - | + | *Run PCR samples 1, 3, & 4 from PCR (E1010 RFP) | |
| - | + | *Add 100bp ladder to two lanes | |
| - | + | ||
| - | + | ||
| + | *Middle three lanes ass 5 µL of PCR product | ||
| + | |||
| + | *Run at 120V for 1 hour | ||
| - | '''May 24, 2012: Transformation of PCR Products in pGEM Vectors in DH5ɑ''' | + | ==='''May 24, 2012: Transformation of PCR Products in pGEM Vectors in DH5ɑ'''=== |
Sample 3 from PCR product ligation | Sample 3 from PCR product ligation | ||
| Line 463: | Line 777: | ||
For each ligation mix: | For each ligation mix: | ||
| - | + | *Added 2µL of ligation mix ( E1010 pcr + pGEM; J23100- mlc + pGEM) to 20.0µL of DH5α | |
| - | + | *Incubated on ice for 30mins | |
| - | + | *45secs in water bath @ 42˚C | |
| - | + | *Immediately incubated on ice for 5mins | |
| - | + | *Added 400µL of SOC media; resuspended | |
| - | + | *Put in shaker for 1hr @ 37 ˚C | |
| - | + | *200µL cultured onto plate(4 plates, Amp) | |
| - | + | *Incubate @ 37 ˚C for approximately 16hrs | |
| + | ==='''May 24 picking colonies of J20100-mlc and E1010 in DH5ɑ'''=== | ||
| - | + | For each sample: | |
| - | + | *Pipette 5 µL of ampicillin into 5 mL LB media | |
| - | + | *Pick colony from plate with a pipette tip and eject tip into media | |
| - | + | *Shake at 37⁰C for approx. 8h | |
| - | |||
| - | |||
| - | - | + | ==='''May 25, 2012: QIA Spin Miniprep of J23100-mlc (A: colony 1), J23100-mlc (B: colony 2)'''=== |
| - | + | *Put 1.5mL of transformed cells in a microcentrifuge tube; centrifuge and repeat until cells from entire tube are in the microcentrifuge tube. | |
| - | + | *Resuspend pelleted bacterial cells in 250uL Buffer P1 | |
| - | + | *Add 250 µL Buffer P2, mix by inversion | |
| - | + | *Add 350 µL Buffer N3, mix by inversion | |
| - | + | *Centrifuge for 10 mins @ 13000 rpm | |
| + | *Put supernatant into the QIAprep spin column | ||
| + | *Centrifuge for 60s at 14000 rpm, discard flow through | ||
| + | *Add 750 µL buffer PE and centrifuge for 60s at 14000 rpm | ||
| + | *Centrifuge again to get rid of remaining buffer | ||
| + | *Add 50 µL of Buffer EB to spin columns, let stand for 1 min, centrifuge for 1 min @ 14000 rpm | ||
| + | ==='''May 26 gel of Miniprep samples'''=== | ||
| + | [[Image:May_26th_miniprep_gel.JPG|left|576px]] | ||
| Line 520: | Line 840: | ||
| - | '''May 29, 2012: Transformation of E1010 fusion in pGEM in DH5ɑ''' | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Gel run to check miniprep samples for DNA | ||
| + | * Ran at 120V for 60 minutes | ||
| + | |||
| + | |||
| + | |||
| + | ==='''May 29, 2012: Transformation of E1010 fusion in pGEM in DH5ɑ'''=== | ||
For each ligation mix: | For each ligation mix: | ||
| - | + | *Added 2µL of ligation mix ( E1010 pcr + pGEM; J23100- mlc + pGEM) to 20.0µL of DH5α | |
| - | + | *Incubated on ice for 30mins | |
| - | + | *45secs in water bath @ 42˚C | |
| - | + | *immediately incubated on ice for 5mins | |
| - | + | *Added 400µL of SOC media; resuspended | |
| - | + | *Put in shaker for 1hr @ 37 ˚C | |
| - | + | *200µL cultured onto plate(4 plates, Amp) | |
| - | + | *Incubate @ 37 ˚C for approximately 16hrs | |
| - | |||
| - | * | + | ==='''May 30 QIA spin Miniprep'''=== |
| + | |||
| + | DNA: | ||
| + | |||
| + | *E1010 psB2k3 | ||
| + | |||
| + | *E1010 fusion pGEM | ||
| + | |||
| + | *J20100-mlc pGEM | ||
| + | |||
| + | *CK331009 psEk3 | ||
| + | |||
| + | *E1010 p5B2k3 | ||
| + | |||
| + | *Put 1.5mL of transformed cells in a microcentrifuge tube; centrifuge and repeat until cells from entire tube are in the microcentrifuge tube. | ||
| + | |||
| + | *Resuspend pelleted bacterial cells in 250µL Buffer P1 | ||
| + | |||
| + | *Add 250 µL Buffer P2, mix by inversion | ||
| + | |||
| + | *Add 350 µL Buffer N3, mix by inversion | ||
| + | |||
| + | *Centrifuge for 10 mins @ 13000 rpm | ||
| + | |||
| + | *Put supernatant into the QIAprep spin column | ||
| + | |||
| + | *Centrifuge for 60s at 14000 rpm, discard flow through | ||
| + | |||
| + | *Add 750 µL buffer PE and centrifuge for 60s at 14000 rpm | ||
| + | |||
| + | *Centrifuge again to get rid of remaining buffer | ||
| + | |||
| + | *Add 50 µL of Buffer EB to spin columns, let stand for 1 min, centrifuge for 1 min at 14000 rpm | ||
==June== | ==June== | ||
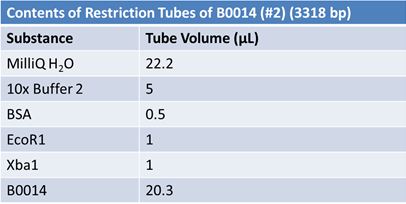
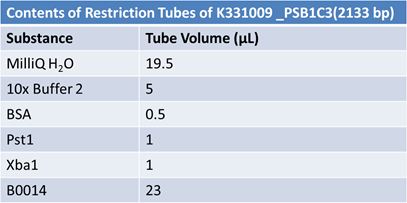
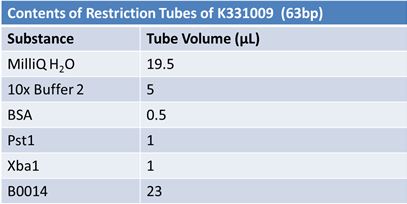
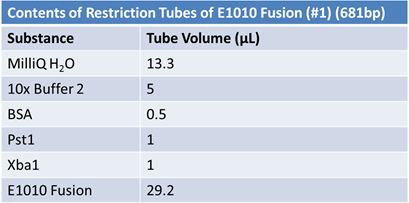
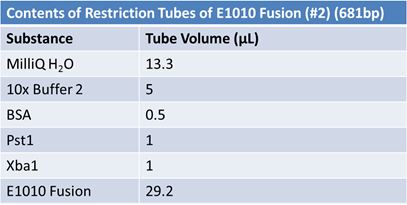
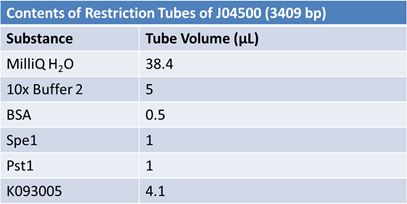
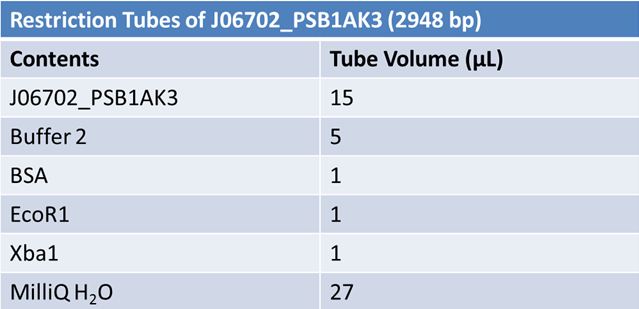
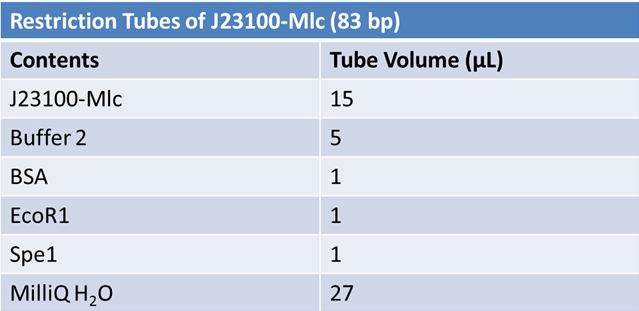
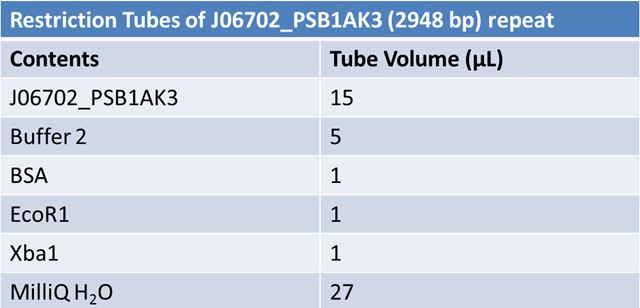
| - | '''June 1, 2012: Restriction of J23100-Mlc, K093005, B0014 (samples | + | ==='''June 1, 2012: Restriction of J23100-Mlc, K093005, B0014 (samples 1 and 2), K331009_PSB1C3, K331009, E1010 Fusion (samples 1 and 2), J04500'''=== |
| + | <br> | ||
| - | + | [[Image:June 1st restriction J23100-Mlc.JPG|left|406px]][[Image:June 1st restriction K093005.JPG|center|406px]] | |
| + | <br> | ||
| + | [[Image:June 1st restriction B0014.JPG|left|406px]][[Image:June 1st restriction B0014 (2).JPG|center|406px]] | ||
| + | <br> | ||
| + | [[Image:June 1st restriction K331009 PSB1C3.JPG|left|407px]][[Image:June 1st restriction K331009.JPG|center|407px]] | ||
| + | <br> | ||
| + | [[Image:June 1st restriction E1010 Fusion.JPG|left|409px]][[Image:June 1st restriction E1010 Fusion (2).JPG|center|409px]] | ||
| + | <br> | ||
| + | [[Image:JUne 1st restriction J04500.JPG|left|407px]] | ||
| + | <br> | ||
| + | [[Image:June1.JPG|center|346px]] | ||
| + | <br> | ||
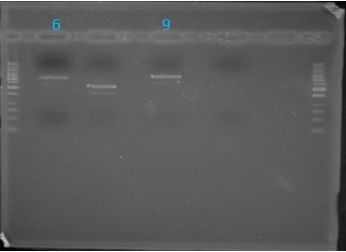
| + | ===Gel Extraction=== | ||
| + | |||
| + | [[Image:June1ge.JPG|left|271px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Only J04500 (lane 6) and K331009 (lane 9) were the expected size | ||
| + | |||
| + | *Restrictions were incubated at 37˚C for 30min then stored in -20˚C | ||
| + | |||
| + | |||
| + | |||
| + | ==='''June 2, 2012: Miniprep of June 1 Cultures'''=== | ||
| + | |||
| + | *Transfer 1.5mL of cell culture to microcentrifuge tube; centrifuge at 5000xg for 2 minutes. Repeat three times. | ||
| + | |||
| + | *Resuspend pelleted cells in 250 µL of Buffer P1 | ||
| + | |||
| + | *Add 250 µL of buffer P2 and mix by inversion | ||
| + | |||
| + | *Add 350 µL of Buffer N1 and mix by inversion | ||
| + | |||
| + | *Centrifuge for 10 minutes at 10,000 rpm | ||
| + | |||
| + | *Apply supernatant to the QIAspin column by decanting | ||
| + | |||
| + | *Centrifuge at 13,000 rpm for 30 seconds | ||
| + | |||
| + | *Wash column by adding 0.75 mL of Buffer PE and centrifuging at 13,000 rpm for 30 seconds | ||
| + | |||
| + | *Discard flow through and centriguge for an additional mintue to remove residual buffer | ||
| + | |||
| + | *To elute DNA, place column in a microcentrifuge tube, add 50 µL of Buffer EV to centre of column, let stand for 1 minute, centrifuge for 1 minute | ||
| + | |||
| + | ==='''June 3, 2012: Confirmaiton of Parts'''=== | ||
| + | |||
| + | [[Image:JUne 3rd size confirmation gel lanes (2).JPG|left|433px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | What worked: | ||
| + | |||
| + | *B0015_PSBIAK3 | ||
| + | |||
| + | *K331009_PSBIC3 | ||
| + | |||
| + | *J04500_PSBIAK3 | ||
| + | |||
| + | *B0015_PSBIAK3 | ||
| + | |||
| + | *J04500_PSBIAK3 | ||
| + | |||
| + | *B0015 _PSBIAK3 | ||
| + | |||
| + | |||
| + | |||
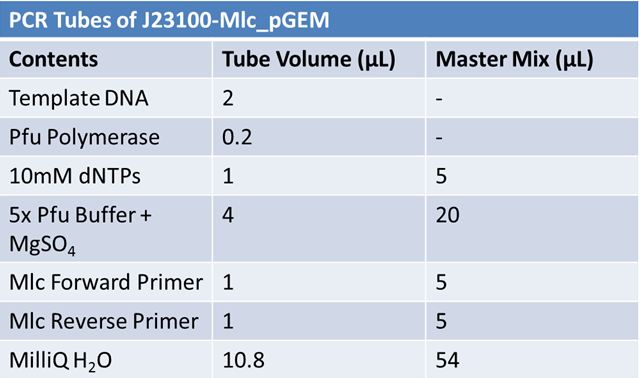
| + | ==='''June 4, 2012: PCR to Confirm J23100-Mlc in pGEM'''=== | ||
| + | |||
| + | [[Image:June 4th PCR J23100-Mlc pGEM.JPG|left|639px]] <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 17.8 µL of the Master Mix to four microcentrifuge tubes. <br> | ||
| + | *Add the template DNA and Pfu polymerase. <br> | ||
| + | |||
| + | |||
| + | |||
| + | ===Transformation of TAT-E1010_pGEM (x2) and J23100-Mlc_pGEM (x1) into DH5α'''=== | ||
| + | |||
| + | For each ligation mix: <br> | ||
| + | *Added 2µL of ligation mix ( E1010 pcr + pGEM; J23100- mlc + pGEM) to 20.0µL of DH5α <br> | ||
| + | *Incubated on ice for 30mins <br> | ||
| + | *Incubated 45secs in water bath @ 42˚C <br> | ||
| + | *Immediately incubated on ice for 5mins <br> | ||
| + | *Added 400µL of SOC media; resuspended <br> | ||
| + | *Put in shaker for 1hr @ 37 ˚C <br> | ||
| + | *200µL cultured onto plate(4 plates, Amp) <br> | ||
| + | *Incubate @ 37 ˚C for approximately 16hrs <br> | ||
| + | |||
| + | ==='''June 5, 2012: Transformation of J06702 (mCherry RFP) into DH5α'''=== | ||
| + | |||
| + | *Add 10µL of MilliQ H2O into Kit Plate (Spring 2011, Kit Plate #2, well E8) | ||
| + | *Added 2µL of DNA to 20.0µL of DH5α <br> | ||
| + | *Incubated on ice for 30mins <br> | ||
| + | *Incubated 45secs in water bath @ 42˚C <br> | ||
| + | *Immediately incubated on ice for 5mins <br> | ||
| + | *Added 400µL of SOC media; resuspended <br> | ||
| + | *Put in shaker for 1hr @ 37 ˚C <br> | ||
| + | *200µL cultured onto plate(4 plates, Amp) <br> | ||
| + | *Incubate @ 37 ˚C for approximately 16hrs <br> | ||
| + | |||
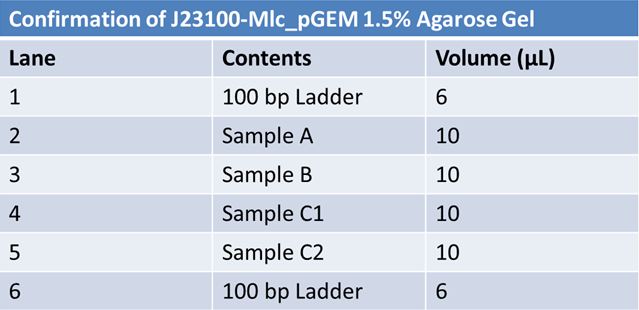
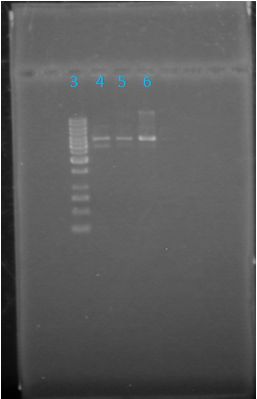
| + | ==='''June 6, 2012: Gel of J23100-Mlc_pGEM Restriction (June 4)'''=== | ||
| + | |||
| + | [[Image:June 6th PCR Gel lanes J23100-Mlc.JPG|left|639px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Sample B worked <br> | ||
| + | |||
| + | |||
| + | |||
| + | ===Miniprep of J06702'''=== | ||
| + | [[Image:June6.JPG|right|257px]] | ||
| + | |||
| + | |||
| + | *Gel confirming J06702 miniprep | ||
| + | |||
| + | *Conformation of part BBa_J06702 | ||
| + | |||
| + | *Miniprep sample cut with EcoR1 and ran on a 1% agarose gel at 120V for 50 mins | ||
| + | |||
| + | *Lanes 4, 5, and 6 contain restricted J06702 in pGEM | ||
| + | |||
| + | |||
| + | *Put 1.5mL of transformed cells in a microcentrifuge tube; centrifuge and repeat until cells from entire tube are in the microcentrifuge tube. <br> | ||
| + | *Resuspend pelleted bacterial cells in 250uL Buffer P1 <br> | ||
| + | *Add 250µL Buffer P2, mix by inversion <br> | ||
| + | *Add 350µL Buffer N3, mix by inversion <br> | ||
| + | *Centrifuge for 10 mins @ 13000 rpm <br> | ||
| + | *Put supernatant into the QIAprep spin column <br> | ||
| + | *Centrifuge for 60s @ 14000 rpm, discard flow through <br> | ||
| + | *Add 750µL buffer PE and centrifuge for 60s @ 14000 rpm <br> | ||
| + | *Centrifuge again to get rid of remaining buffer <br> | ||
| + | *Add 50µL of Buffer EB to spin columns, let stand for 1 min, centrifuge for 1 min at 14000 rpm <br> | ||
| + | |||
| + | |||
| + | *3 samples were made and ran on a gel, all samples appeared to be the expected size | ||
| + | |||
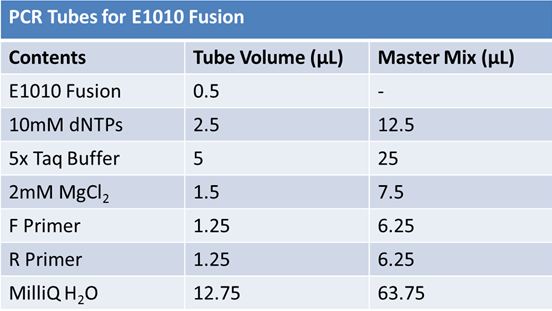
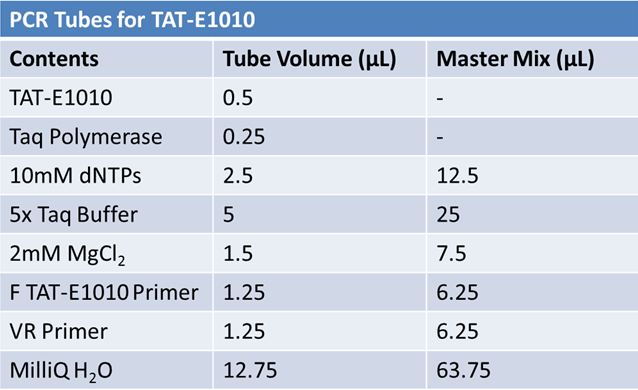
| + | ===PCR of E1010 Fusion (to confirm part) and TAT-E1010 (for insertion into pGEM)'''=== | ||
| + | |||
| + | [[Image:June 6th PCR tubes E1010 fusion.JPG|left|638px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 18.8 µL of the Master Mix into 4 microcentrifuge tubes <br> | ||
| + | *Add E1010 Fusion and Pfu polymerase <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:June 6th PCR tubes TAT-E1010.JPG|left|638px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:June 6th PCR Gel lanes TAT-E1010.JPG|left|px]][[Image:June6tat.JPG|center|244px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Add 24.25 µL of the Master Mix into 4 microcentrifuge tubes | ||
| + | *Add TAT-E1010 and Taq polymerase | ||
| + | |||
| + | |||
| + | |||
| + | ===Size Confirmation Gel of E1010 fusion, TAT-E1010, J23100-Mlc, J04500'''=== | ||
| + | |||
| + | [[Image:June 6th size confirmaiton gel lanes.JPG|left|584px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *All TAT-E1010 samples worked | ||
| + | * Sample from lane 5 was ligated with pGEM | ||
| + | |||
| + | ===June 7th 2012: Transformation of TAT-E1010_pGEM (x2) and J23100-mlc_pGEM into DH5ɑ=== | ||
| + | |||
| + | *Added 2.0 µL of DNA to 20 µL of DH5ɑ. | ||
| + | |||
| + | *Incubated on ice for 30 minutes. | ||
| + | |||
| + | *Placed in water bath for 45 seconds at 42⁰C. | ||
| + | |||
| + | *Immediately incubated in ice for 5 minutes. | ||
| + | |||
| + | *Added 400µL of SOC media, re-suspended. | ||
| + | |||
| + | *Shaked for one hour @ 37⁰C. | ||
| + | |||
| + | *While shaking, 40µL of 0.1M IPTG and 40ul X-Gal was added to each plate | ||
| + | |||
| + | *Added 200µL onto plate. | ||
| + | *Incubated for approximately 16 hours at 37⁰C. | ||
| + | |||
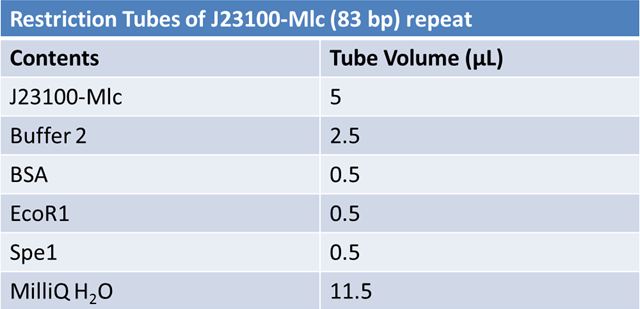
| + | ===June 10th 2012: Restriction of J23100-mlc with E and X and J06702 with S and P and Ligation of J23100-mlc with J06702 (pSB1A2) === | ||
| + | |||
| + | '''Restriction:''' | ||
| + | |||
| + | [[Image:June 10th restriction tubes J06702 PSB1AK3(1).JPG|left|px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:June_10th_restriction_tubes_J23100-Mlc.JPG|left|px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Ran samples on a 2% agarose gel at 120V for 1 hour. <br> | ||
| + | *Only J23100 was seen at the expected size and was extracted from gel according to standard protocol. <br> | ||
| + | *Restrictions were repeated.<br> | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:June_10th_restriction_J06702_repeat.JPG |left|px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:JUne_10th_restriction_J23100_repeat.JPG|left|px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Restriced at 37⁰C for 1 hour.<br> | ||
| + | |||
| + | |||
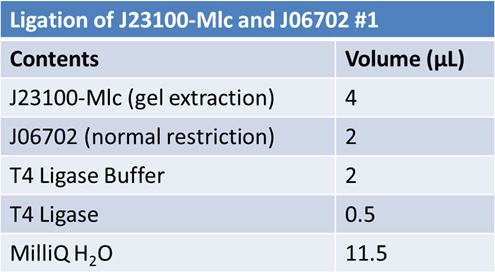
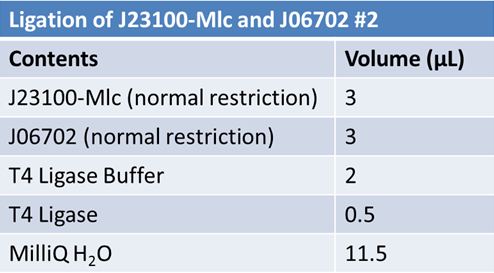
| + | '''Ligation:''' | ||
| + | |||
| + | [[Image:June 10th ligation 1.JPG|left|px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:June 10th ligation 2.JPG|left|px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ===June 11th 2012: Transformation of J23100-mlc-J06702 samples from June 10th=== | ||
| + | |||
| + | For each ligation mix: | ||
| + | |||
| + | *Added 2µL of ligation mix to 20.0µL of DH5α | ||
| + | |||
| + | *Incubated on ice for 30mins | ||
| + | |||
| + | *45secs in water bath @ 42˚C | ||
| + | |||
| + | *immediately incubated on ice for 5mins | ||
| + | |||
| + | *Added 400µL of SOC media; resuspended | ||
| + | |||
| + | *Put in shaker for 1hr @ 37 ˚C | ||
| + | |||
| + | *200µL cultured onto plate(4 plates, Amp) | ||
| + | |||
| + | *Incubate @ 37 ˚C for approximately 16hr | ||
| + | |||
| + | |||
| + | *4 plates (according to June 10th ligation): | ||
| + | |||
| + | *A | ||
| + | |||
| + | *B | ||
| + | |||
| + | *C | ||
| + | |||
| + | *D | ||
| + | |||
| + | |||
| + | *2x J23100-m/c (gel extraction) Amp. | ||
| + | |||
| + | *2x J06702 Amp. | ||
| + | |||
| + | *Added 2.0 microlitres, mixed to 20 microlitres of DH5 Alpha. | ||
| + | |||
| + | *Incubated on ice for 30 minutes. | ||
| + | |||
| + | *Placed in water bath for 45 seconds at 42 degrees Celsius. | ||
| + | |||
| + | *Immediately incubated in ice for 5 minutes. | ||
| + | |||
| + | *Added 400 microlitres of SOC media, re-suspended. | ||
| + | |||
| + | *Shook for one hour @ 37 degrees Celsius. | ||
| + | |||
| + | *Added 200 microlitres onto plate. | ||
| + | |||
| + | *Incubated for approximately 16 hours at 37 degrees Celsius. | ||
| + | |||
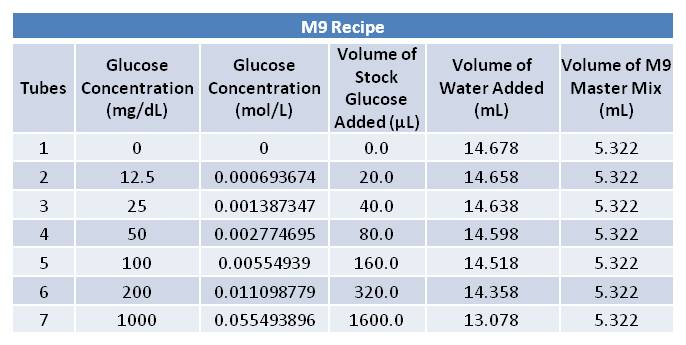
| + | ===June 12th, 2012: Picking Cells and Making M9 Media=== | ||
| + | |||
| + | |||
| + | *Colonies grew on all 4 plates | ||
| + | |||
| + | *Cells were picked from each plate and put into 5ml LB flasks with 5ul Ampicillin each | ||
| + | |||
| + | *1L M9 media made as per Knight protocol [http://openwetware.org/wiki/Knight:M9_supplemented_media] | ||
| + | |||
| + | Aliquoted as two 10 mL samples of the same glucose concentration. Replacing a portion of the water added with various volumes of 0.693674 mol/L glucose solution as follows: | ||
| + | |||
| + | [[File:M9 pic.jpg]] | ||
| + | |||
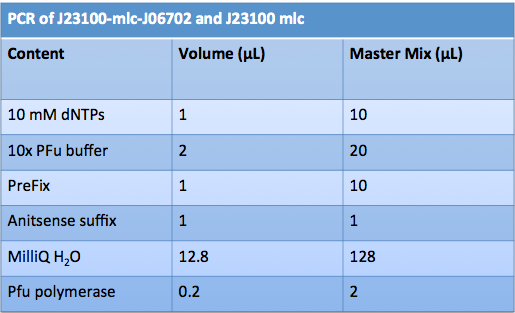
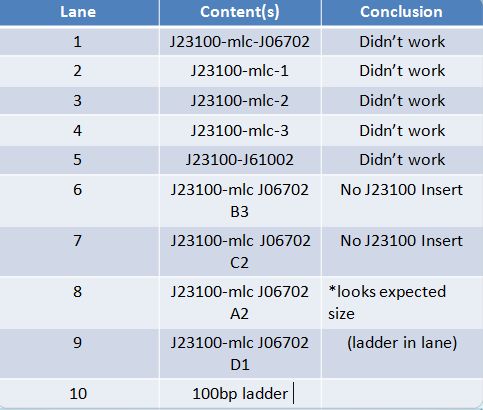
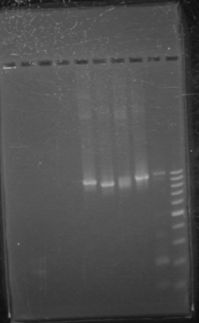
| + | ==='''June 14th, 2012 PCR of J23100-Mlc-J06702 and J23100-Mlc'''=== | ||
| + | |||
| + | [[Image:Screen_Shot_2012-06-16_at_5.58.39_PM.png|left"px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:June14.JPG|left|483px]] | ||
| + | |||
| + | [[Image:June14gel.JPG|center|199px]]<br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
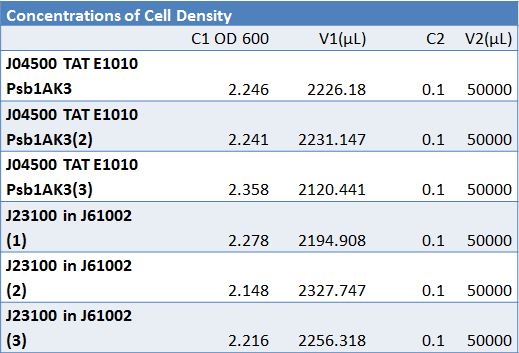
| + | ==='''June 15, 2012:Transformation of Plasmid PSB1C3 with J23100-Mlc and K093005 and Insulin Secretion Proof of Princliple'''=== | ||
| + | |||
| + | ===Transformation:'''=== | ||
| + | |||
| + | *Add 20 μL of ligation mix to 20 μL of DH5α | ||
| + | |||
| + | *Incubate on ice for 30 minutes | ||
| + | |||
| + | *30 second s in water bath at 42⁰C | ||
| + | |||
| + | *Immediately incubate in ice for 5 minutes | ||
| + | |||
| + | *Add 200μL of SOC media;resuspend | ||
| + | |||
| + | *Shake for 1 hour at 37oC for approx. 16 hours | ||
| + | |||
| + | ===Insulin Secretion Proof of Principle'''=== | ||
| + | |||
| + | Picked 4 cultures from -80 freezer stocks: <br> | ||
| + | *2 -J04500 + TAT –E1010 <br> | ||
| + | *2 – J23100 in J61002<br> | ||
| + | Cultures were grown over night <br> | ||
| + | The following morning: <br> | ||
| + | *The 5ml cultures were spun down and re-suspended in one milliliter of fresh media.<br> | ||
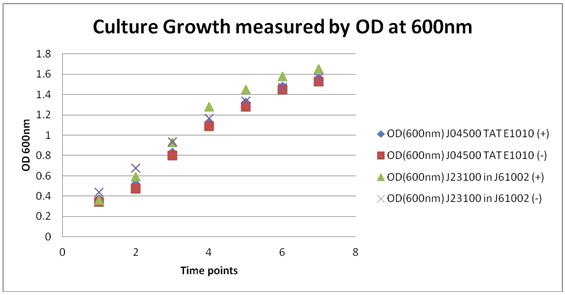
| + | *The OD was taken at 600nm and where recorded to determine the amount of the re-suspended culture was need to inoculate 50ml of media. <br> | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:POP Concentrations of Cell Densities.JPG|left|px]] | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | (Note not enough culture was available so 500µL of the 1000µL of the 3rd culture was split between each new culture) | ||
| + | |||
| + | The re-suspended cells were added to 50 ml cultures and 50ul of Ampicillin was also added to media. Cultures were placed in the shaker and shaken at 37 ⁰C. <br> | ||
| + | Cultures were measured at 600nm for OD at 1 hour time points from the beginning of shaking.<br> | ||
| + | |||
| + | |||
| + | [[Image:Culture growth measured by OD at 600nm.JPG|left|px]] | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Culture Samples were spun down and at 8000rpm for 2 minutes and supernatant was taken and ran through a fluorescent spectrophotometer for excitation of RFP at 586nm and expected emission at 607nm. | ||
| + | |||
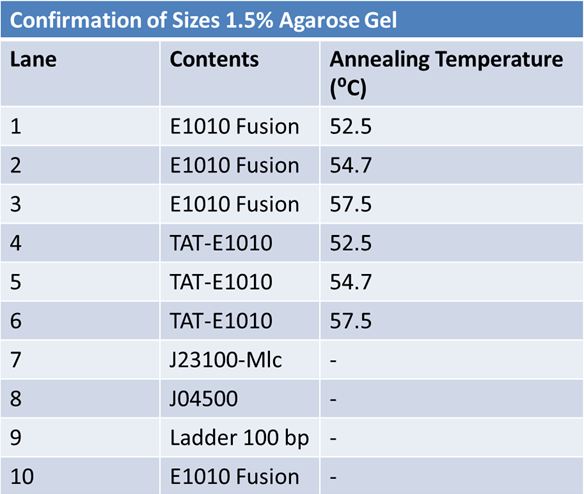
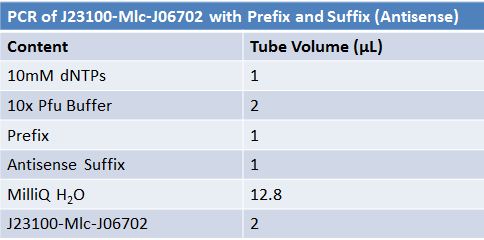
| + | ==='''June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)'''=== | ||
| + | |||
| + | |||
| + | [[Image:June 16 PCR J23100-Mlc-J06702.JPG|left|px]] | ||
| - | + | (See [https://2012hs.igem.org/Team:Lethbridge_Canada/Results "Results"].) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 03:53, 17 June 2012

| Home | The Team | The Project | Results | Human Practices | Notebook | Safety |
|---|
NotebookMarchBrainstorming Project Ideas
The chosen project
Project outlinePossible things to consider:
AprilSub-groupsWe divided the team into sub-groups. Each group assumed responsibility for a different aspect of the project. Wiki and Mascot Design This group is responsible to obtaining and uploading all necessary information to the wiki. This includes team pictures and biographies, experiments and results, and the projects of the other sub-groups. This will be a priority over mascot design.Leader: Erin Kelly
This group must document the safety protocols that the Lethbridge team practices in the lab.Leader: Marissa Guzzi
This group is responsible for letting the public know what our team is and what we are doing. This can be done creatively. The point is to raise awareness for iGEM, synthetic biology, and diabetes.Leader: Chris Isaac WHMIS tests and Safety TrainingWe are required to have safety training in order to work in the lab, and so we all wrote our WHMIS tests and did our Hazards Assesment and lab orientation! MayMay 8, 2012: Tansformation of Parts from Kit Plate and Growing Cells from Glycerol StocksTransformation of parts from kit plate:
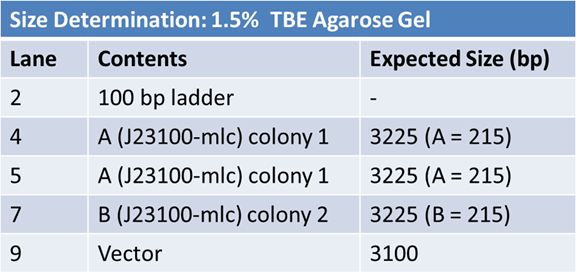
May 9, 2012: Miniprep and Restriction of PartsOvernight cultures from May 8th were miniprepped and restricted in order to determine sizes.
Master mix was prepared by mixing the ingredients together in order, as shown above. 20µL worth of master mix was combined with 5µL of plasmid DNA:
Restrictions were incubated at 37˚C for 30mins then stored at -20˚C.
May 10, 2012: Miniprep and Restriction of PartsOvernight cultures from May 9th were miniprepped and restricted in order to determine sizes
Restrictions were incubated at 37˚C for 30min then stored in -20˚C.
May 11th: Electrophoresis of restricted parts from May 10th
Purpose: confirm existence of the following parts:
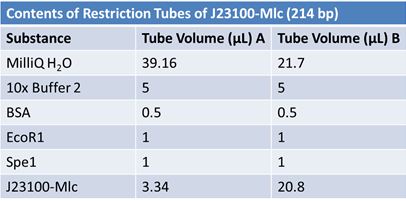
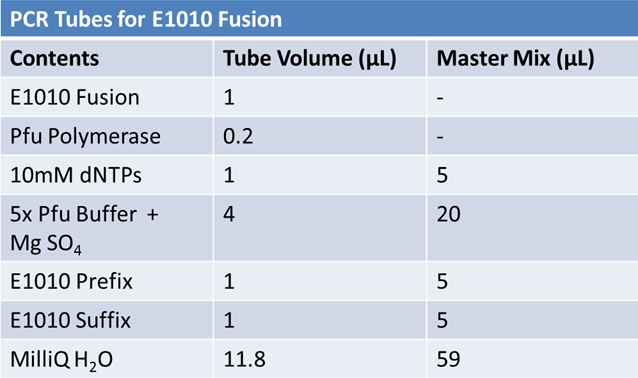
PCR of RFP (E1010) into fusion standard (adding extra bp to get into fusion standard) Add 17.8 µL to 8 tubes with: May 12th: Assembly of promoter & RBS (J04500) with signal sequence (K331009) and RPF (E1010 fusion) with double terminator (B0015)Method: Restriction, gel extraction, and ligation Restriction:
We made a 1% TAE agarose gel and ran restrictions for 1 hour @ 120V We then gel extracted the DNA Ligation:
May 13th Transformation of ligated products: J04500 + K331009 and E1010 (fusion) + B0015Transformation: 2µL of each ligation mix was added to 20µL DH5ɑ cells
May 14th: Picking Cells from May 13th transformation
May 19th: PCR of E1010 and J23100 (Mlc primers)E1010:
Ran 1.5% agarose TAE gel at 90V for 45 mins
May 20: Re-do May 19 PCR of E1010 (fusion) and J23100-mlcAlterations to PCR:
PCR cycle:
May 21st: PCR of J23100 and E1010 repeat again!
For both J20100 and E1010:
PCR protocol:
May 22, 2012: PCR of J23100-MlcPCR Parameters: Initial denaturation 95˚C 3mins 30 cycles of: *denaturation 95˚C 30s *annealing (various) 30s *elongation 72˚C 1min
1.5% agarose gel Ran at 120v for 1 hr
May 23, 2012: Ligation of PCR products into pGEM Vector
2) Prefix-(fusion)-E1010-suffix (Black tubes) (sample 1 from May 23rd PCR) Contents:
May 23, 2012 Transformation of J23100-Mlc and E1010 fusionFor each ligation mix:
-0.45 agarose M -30 mL TAE (1x)
May 24, 2012: Transformation of PCR Products in pGEM Vectors in DH5ɑSample 3 from PCR product ligation For each ligation mix:
May 24 picking colonies of J20100-mlc and E1010 in DH5ɑFor each sample:
May 25, 2012: QIA Spin Miniprep of J23100-mlc (A: colony 1), J23100-mlc (B: colony 2)
May 26 gel of Miniprep samples
May 29, 2012: Transformation of E1010 fusion in pGEM in DH5ɑFor each ligation mix:
May 30 QIA spin MiniprepDNA:
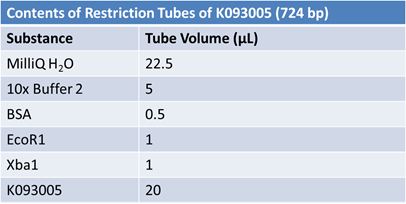
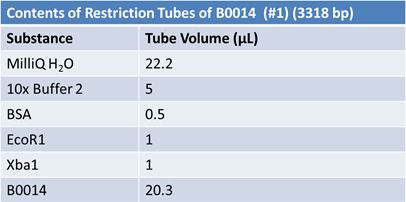
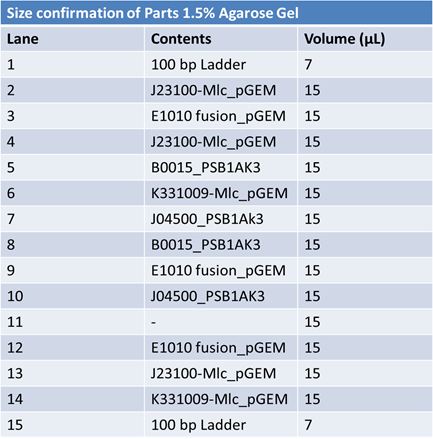
JuneJune 1, 2012: Restriction of J23100-Mlc, K093005, B0014 (samples 1 and 2), K331009_PSB1C3, K331009, E1010 Fusion (samples 1 and 2), J04500
Gel Extraction
June 2, 2012: Miniprep of June 1 Cultures
June 3, 2012: Confirmaiton of Parts
What worked:
June 4, 2012: PCR to Confirm J23100-Mlc in pGEM
Transformation of TAT-E1010_pGEM (x2) and J23100-Mlc_pGEM (x1) into DH5αFor each ligation mix:
June 5, 2012: Transformation of J06702 (mCherry RFP) into DH5α
June 6, 2012: Gel of J23100-Mlc_pGEM Restriction (June 4)
Miniprep of J06702
PCR of E1010 Fusion (to confirm part) and TAT-E1010 (for insertion into pGEM)
Size Confirmation Gel of E1010 fusion, TAT-E1010, J23100-Mlc, J04500
June 7th 2012: Transformation of TAT-E1010_pGEM (x2) and J23100-mlc_pGEM into DH5ɑ
June 10th 2012: Restriction of J23100-mlc with E and X and J06702 with S and P and Ligation of J23100-mlc with J06702 (pSB1A2)Restriction:
June 11th 2012: Transformation of J23100-mlc-J06702 samples from June 10thFor each ligation mix:
June 12th, 2012: Picking Cells and Making M9 Media
Aliquoted as two 10 mL samples of the same glucose concentration. Replacing a portion of the water added with various volumes of 0.693674 mol/L glucose solution as follows: June 14th, 2012 PCR of J23100-Mlc-J06702 and J23100-Mlc
June 15, 2012:Transformation of Plasmid PSB1C3 with J23100-Mlc and K093005 and Insulin Secretion Proof of PrinclipleTransformation:
Insulin Secretion Proof of PrinciplePicked 4 cultures from -80 freezer stocks:
Cultures were grown over night
The re-suspended cells were added to 50 ml cultures and 50ul of Ampicillin was also added to media. Cultures were placed in the shaker and shaken at 37 ⁰C.
June 16, 2012: PCR of J23100-Mlc-J06702 with Prefix and Suffix (Antisense)(See "Results".) |
 "
"