Team:Lethbridge Canada/Results
From 2012hs.igem.org
(→Proof of Principle) |
(→Glucose Concentration Assay) |
||
| (20 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
</html> | </html> | ||
{| style="color:#000000;background-color:#b5cde2;" cellpadding="6" cellspacing="3" border="0" bordercolor="#00FFFF" width="65%" align="center" | {| style="color:#000000;background-color:#b5cde2;" cellpadding="6" cellspacing="3" border="0" bordercolor="#00FFFF" width="65%" align="center" | ||
| - | !align="center"|[[Image: | + | !align="center"|[[Image:Project.JPG|center|897px]] |
|} | |} | ||
| Line 30: | Line 30: | ||
='''Results'''= | ='''Results'''= | ||
| - | ==Parts | + | <div align="center"> |
| + | |||
| + | ==Parts Created== | ||
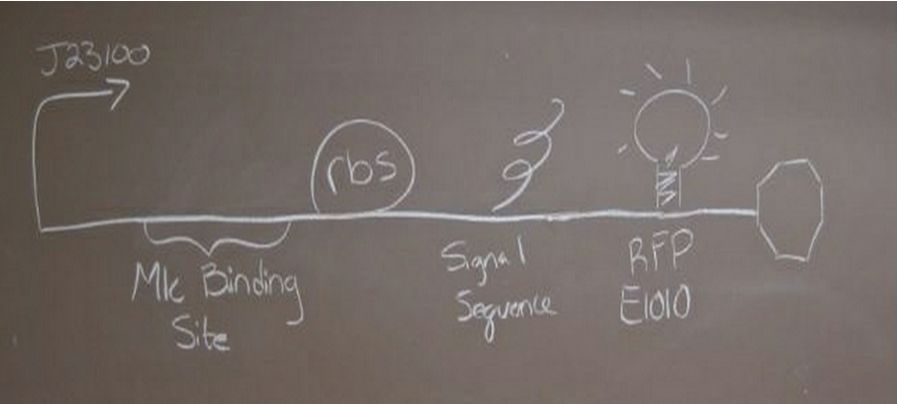
We created two new parts, [http://partsregistry.org/Part:BBa_K736000 BBa_K736000] (which regulated the transcription of downstream genes) and [http://partsregistry.org/Part:BBa_K736001 BBa_K736001] (which was a TAT signal sequence fused to a red fluorescent protein, E1010). We also assembled two parts, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736002 BBa_K736002] (which tests the ability of glucose to regulate transcription and uses enhanced RFP as the reporter) and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736003 BBa_K736003] (which tests for the ability of the TAT sequence to cause secretion of protein outside of the cell and also uses RFP as a reporter). We also created one part that coded for human insulin chain A ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736004 BBa_K736004]) and one part that coded for human insulin chain B ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736005 BBa_K736005]). We are also submitting the DNA for [http://partsregistry.org/Part:BBa_K331009 K331009] which was in its planning stage in the registry. | We created two new parts, [http://partsregistry.org/Part:BBa_K736000 BBa_K736000] (which regulated the transcription of downstream genes) and [http://partsregistry.org/Part:BBa_K736001 BBa_K736001] (which was a TAT signal sequence fused to a red fluorescent protein, E1010). We also assembled two parts, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736002 BBa_K736002] (which tests the ability of glucose to regulate transcription and uses enhanced RFP as the reporter) and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736003 BBa_K736003] (which tests for the ability of the TAT sequence to cause secretion of protein outside of the cell and also uses RFP as a reporter). We also created one part that coded for human insulin chain A ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736004 BBa_K736004]) and one part that coded for human insulin chain B ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736005 BBa_K736005]). We are also submitting the DNA for [http://partsregistry.org/Part:BBa_K331009 K331009] which was in its planning stage in the registry. | ||
| Line 50: | Line 52: | ||
| - | + | <br/> | |
| + | |||
| + | =='''Insulin Red Fluorescent Protein Proof of Principle'''== | ||
TAT signal sequence | TAT signal sequence | ||
'''RFP with Cells Media at Different Induction Levels''' | '''RFP with Cells Media at Different Induction Levels''' | ||
| + | |||
Preliminary results | Preliminary results | ||
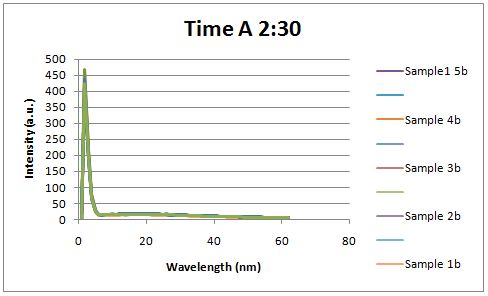
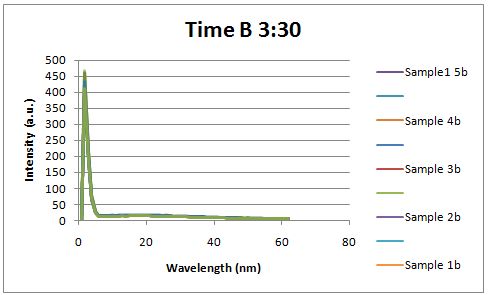
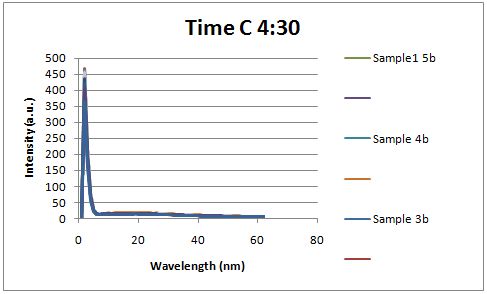
| - | Cultures were grown containing | + | Cultures were grown containing LacI promoter attached to a twin arginine tag (TAT) signal sequence and fused to a red fluorescent protein (E1010). Samples were taken at various time points and examined for fluorescence when excited at 586nm with an expected emission at 607nm. We used a fluorescence spectrophotometer to measure the red fluorescent protein present in the supernatant after spinning the cells to a pellet. We used a constutively expressed construct expressing Red fluorescent protein( J23100 in J61002) |
<br> | <br> | ||
[[Image:Time A.JPG|left|px]] | [[Image:Time A.JPG|left|px]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | |||
| - | |||
| - | + | ||
| - | + | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Fluorescence was measured one hour after induction, no difference could be seen between the test and control constructs in the supernatant . | ||
<br> | <br> | ||
| Line 86: | Line 90: | ||
| + | [[Image:Time B.JPG|left|px]] | ||
| + | <br> | ||
| Line 92: | Line 98: | ||
| + | Fluorescence was measured two hours after induction, no difference could be seen between the test and control constructs in the supernatant . | ||
| Line 101: | Line 108: | ||
| + | <br> | ||
| + | [[Image:Time C.JPG|left|px]] | ||
| + | <br> | ||
| Line 111: | Line 121: | ||
| + | Fluorescence was measured three hours after induction, no difference could be seen between the test and control constructs in the supernatant . | ||
| Line 117: | Line 128: | ||
| + | <br> | ||
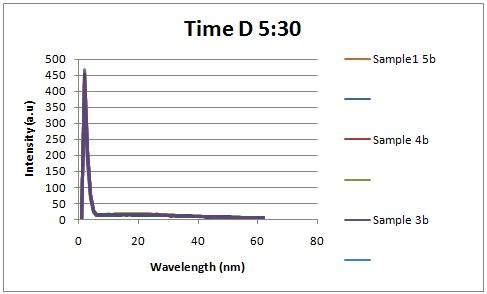
| + | [[Image:Time D.JPG|left|px]] | ||
| Line 125: | Line 138: | ||
| + | <br> | ||
| + | Fluorescence was measured four hours after induction, no difference could be seen between the test and control constructs in the supernatant . | ||
| + | <br> | ||
| Line 144: | Line 160: | ||
| + | ==Glucose Concentration Assay== | ||
| + | Preliminary Results | ||
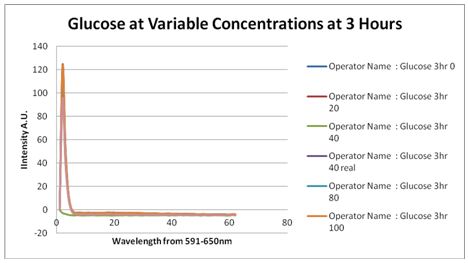
| + | Cultures were grown containing constitutively expressed promoter J23100 attached to an MlC binding sight sequence and a red fluorescent protein. Samples were inoculated in varying concentrations of Glucose (0=0 mg/dl, 20= 12.5mg/dl, 40=25mg/dl, 80=50mg/dl, 160=100mg/dl, 320=200mg/dl, 1600=1000mg/dl). Samples were taken after 1.5 hour time points post inoculation and examined for fluorescents when excited at 586nm with an expected emission at 607nm. We used a fluorescents spectrophotometer to measure the red fluorescent protein present in cell lysate after spinning the cells to a pellet and re-suspending in 8M urea. | ||
| + | <br> | ||
| + | [[Image:Glucose assay 3 hour time point.JPG|left|px]] | ||
| + | <br> | ||
| Line 156: | Line 178: | ||
| + | Fluorescence was measured three hours after inoculation, no RFP emission differences between cells grown in the different glucose concentrations were observed. | ||
| + | <br> | ||
| Line 162: | Line 186: | ||
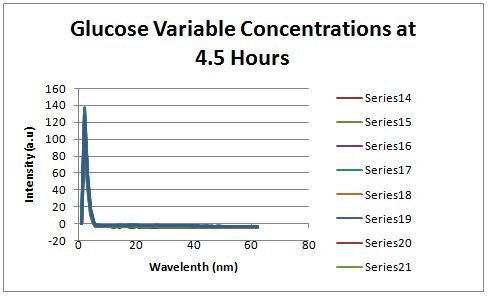
| + | [[Image:Glucose variable concentrations 4.5 hours.JPG|left|px]] | ||
| + | <br> | ||
| Line 173: | Line 199: | ||
| + | Fluorescence was measured four and a half hours after inoculation, no RFP emission differences between cells grown in the different glucose concentrations were observed. | ||
| + | <br> | ||
| Line 181: | Line 209: | ||
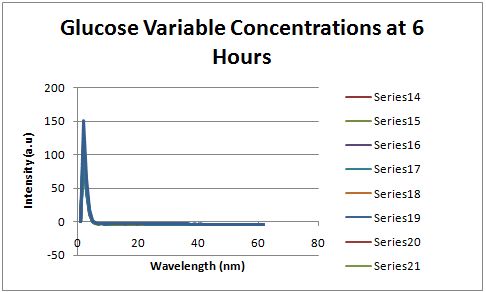
| + | [[Image:Glucose variable concentrations 6 hours.JPG|left|px]] | ||
| - | + | <br> | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Fluorescence was measured six hours after innoculation, no RFP emission differences between cells grown in the different glucose concentrations were observed. | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
Latest revision as of 07:58, 17 June 2012

| Home | The Team | The Project | Results | Human Practices | Notebook | Safety |
|---|
ResultsParts CreatedWe created two new parts, [http://partsregistry.org/Part:BBa_K736000 BBa_K736000] (which regulated the transcription of downstream genes) and [http://partsregistry.org/Part:BBa_K736001 BBa_K736001] (which was a TAT signal sequence fused to a red fluorescent protein, E1010). We also assembled two parts, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736002 BBa_K736002] (which tests the ability of glucose to regulate transcription and uses enhanced RFP as the reporter) and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K736003 BBa_K736003] (which tests for the ability of the TAT sequence to cause secretion of protein outside of the cell and also uses RFP as a reporter). We also created one part that coded for human insulin chain A ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736004 BBa_K736004]) and one part that coded for human insulin chain B ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K736005 BBa_K736005]). We are also submitting the DNA for [http://partsregistry.org/Part:BBa_K331009 K331009] which was in its planning stage in the registry.
Insulin Red Fluorescent Protein Proof of PrincipleTAT signal sequence RFP with Cells Media at Different Induction Levels Preliminary results Cultures were grown containing LacI promoter attached to a twin arginine tag (TAT) signal sequence and fused to a red fluorescent protein (E1010). Samples were taken at various time points and examined for fluorescence when excited at 586nm with an expected emission at 607nm. We used a fluorescence spectrophotometer to measure the red fluorescent protein present in the supernatant after spinning the cells to a pellet. We used a constutively expressed construct expressing Red fluorescent protein( J23100 in J61002)
Glucose Concentration AssayPreliminary Results Cultures were grown containing constitutively expressed promoter J23100 attached to an MlC binding sight sequence and a red fluorescent protein. Samples were inoculated in varying concentrations of Glucose (0=0 mg/dl, 20= 12.5mg/dl, 40=25mg/dl, 80=50mg/dl, 160=100mg/dl, 320=200mg/dl, 1600=1000mg/dl). Samples were taken after 1.5 hour time points post inoculation and examined for fluorescents when excited at 586nm with an expected emission at 607nm. We used a fluorescents spectrophotometer to measure the red fluorescent protein present in cell lysate after spinning the cells to a pellet and re-suspending in 8M urea.
Fluorescence was measured six hours after innoculation, no RFP emission differences between cells grown in the different glucose concentrations were observed.
|
 "
"