Team:Dalton School NY/Notebook
From 2012hs.igem.org
(→Obtaining the remaining clones) |
|||
| Line 67: | Line 67: | ||
TEF1 '''none''' | TEF1 '''none''' | ||
| - | EAF6 '''none''' | + | EAF6 '''none''' (possibly a band, but too little DNA to be confident) |
HSP12 yes: A, B | HSP12 yes: A, B | ||
| Line 116: | Line 116: | ||
BsaI digests were incubated at 37°C for 2 hrs and then run on a 2% agarose gel in 1X sodium borate buffer. | BsaI digests were incubated at 37°C for 2 hrs and then run on a 2% agarose gel in 1X sodium borate buffer. | ||
| + | |||
| + | |||
| + | [[Image:Dalton_Fig9.jpg]] | ||
| + | |||
| + | |||
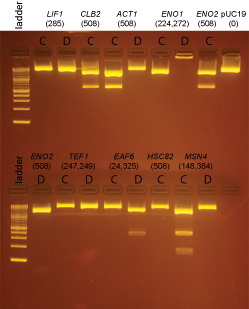
| + | Based on the data above, we concluded that the following clones had inserts of the correct size: | ||
| + | |||
| + | '''Promoters''' | ||
| + | |||
| + | LIF1 '''none''' | ||
| + | |||
| + | CLB2 yes: C | ||
| + | |||
| + | ACT1 yes: C | ||
| + | |||
| + | ENO1 '''none''' | ||
| + | |||
| + | ENO2 yes: C | ||
| + | |||
| + | TEF1 '''none''' | ||
| + | |||
| + | EAF6 yes: D (This was started from culture EAF6-B) | ||
| + | |||
| + | HSC82 '''none''' | ||
| + | |||
| + | MSN4 '''none''' | ||
| + | |||
Return to our [https://2012hs.igem.org/Team:Dalton_School_NY main page]. | Return to our [https://2012hs.igem.org/Team:Dalton_School_NY main page]. | ||
Revision as of 03:12, 1 June 2012
In progress...
Contents |
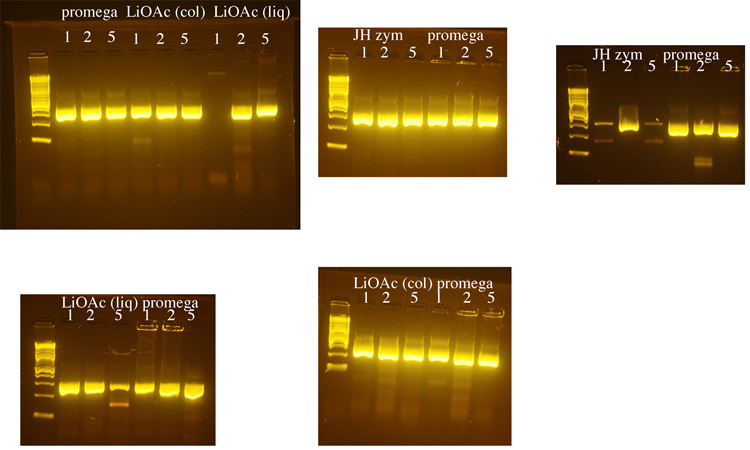
Testing of DNA prepared by several methods
Yeast genomic DNA is commonly prepared using a phenol/chloroform based method. Because both phenol and chloroform are toxic chemicals, we tested several methods for preparing yeast genomic DNA that do not use these chemicals. We compared a method that uses LiOAc to disrupt the yeast cell wall (BioTechniques 50:325-328, 2011) to methods that use the enzyme zymolyase to disrupt the cell wall (a Promega kit or a protocol from Jim Haber’s lab, “JH zym”). The Haber lab protocol produced the greatest yield of genomic DNA, but DNA made using the Promega kit was used in subsequent PCRs because it seemed to produce the most consistent results. 1µl, 2µl, or 5µl of genomic DNA was used in each PCR reaction with LIF1 promoter primers. Following these initial tests, 1µl of yeast genomic DNA was used for subsequent PCRs. The LiOAc method was the shortest and least expensive and we may switch to this method when future DNA preps are needed.
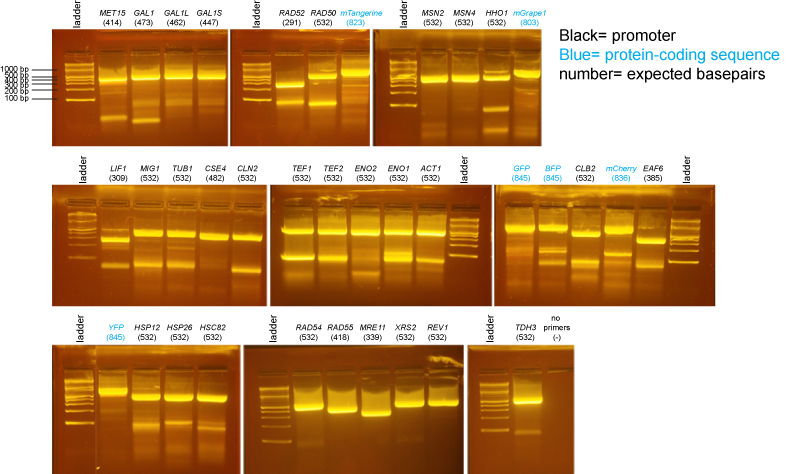
PCR of promoters and fluorescent protein coding sequences
BsaI-digested Miniprep DNA
The samples on these gels contain BsaI-digested miniprep DNA prepared from individual colonies from the transformations above.
Based on the data above, we concluded that the following clones had inserts of the correct size:
Promoters
MET15 yes: B
GAL1 yes: A
GAL1-L yes: A
GAL1-S yes: B
RAD52 yes: B
RAD50 yes: A
RAD54 yes: A, B
RAD55 yes: A, B
MRE11 yes: A, B
XRS2 yes: A, B
REV1 yes: B
TDH3 yes: A, B
LIF1 none
MIG1 yes: B
TUB1 yes: A
CSE4 yes: B
CLN2 yes: B
CLB2 none
ACT1 none
ENO1 none
ENO2 none
TEF2 yes: B
TEF1 none
EAF6 none (possibly a band, but too little DNA to be confident)
HSP12 yes: A, B
HSP26 yes: A
HSC82 none
MSN2 yes: A
MSN4 none
HHO1 yes: A, D
Protein-coding sequences
BFP maybe: A (the insert may be too large)
GFP none
YFP none
mTangerine none
mCherry yes: A (but bottom band seems possibly too intense)
mGrape1 none
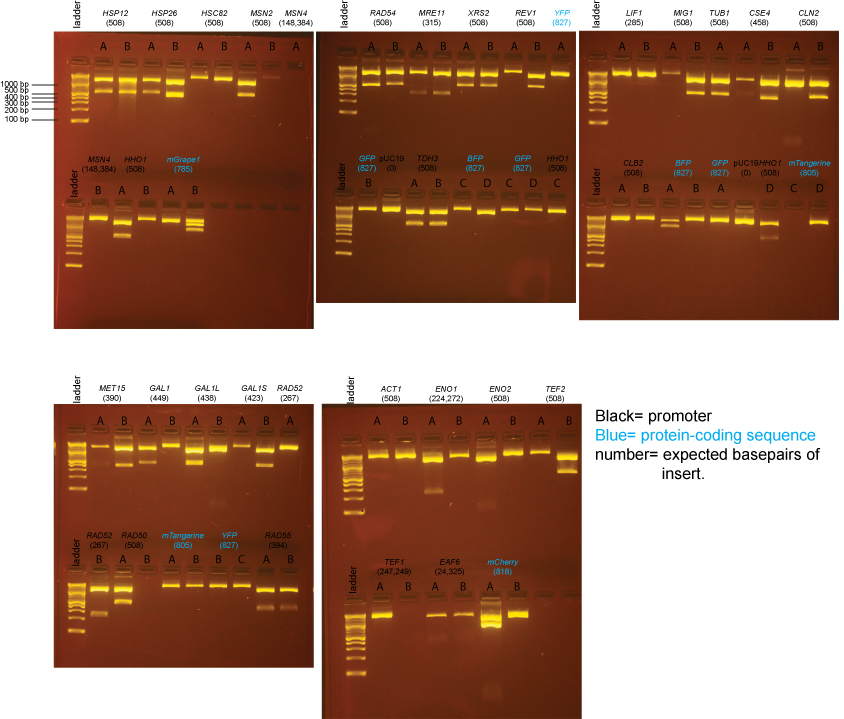
Obtaining the remaining clones
Extra colonies were picked from the transformations for the following promoter clones:
LIF1, CLB2, ACT1, ENO1, ENO2, TEF1, EAF6, HSC82, MSN4
DNA from these clones was miniprepped using Qiagen minipreps. The DNA was digested with BsaI as follows:
miniprep DNA: 3µl
10X NEBuffer 4: 2µl
100X BSA: 0.2µl
BsaI: 0.5µl
H2O: 14.8µl
BsaI digests were incubated at 37°C for 2 hrs and then run on a 2% agarose gel in 1X sodium borate buffer.
Based on the data above, we concluded that the following clones had inserts of the correct size:
Promoters
LIF1 none
CLB2 yes: C
ACT1 yes: C
ENO1 none
ENO2 yes: C
TEF1 none
EAF6 yes: D (This was started from culture EAF6-B)
HSC82 none
MSN4 none
Return to our main page.
 "
"