Team:CSIA SouthKorea/protocols
From 2012hs.igem.org
- Our protocol is based on 3A assembly protocol provided by iGEM. Standard assembly is divided into 'growing', 'miniprep', 'restriction digest', 'ligation' and 'transformation'. The following texts are from partsregistry.
Restriction Digest
- 1. Clean the lab bench by wiping down with 70% ethanol and paper towels. Keep all enzymes and buffers used in this section on ice.
- 2. Label four 0.6 tubes: Part A, Part B, pSB1C3 (linearized plasmid backbone), and RFP Control
-
- 3. Add 500ng of DNA to the appropriate tube.
- 4. Pipet 5ul of Buffer 2 to each tube.
- 5. Pipet 0.5ul of BSA to each tube.
- 6. In the Part A tube: Add 1ul of EcoRI enzyme, and 1ul of SpeI enzyme.
- In the Part B tube: Add 1ul of XbaI enzyme, and 1ul of PstI enzyme.
- In the pSB1C3 tube: Add 1ul of EcoRI enzyme, and 1ul of PstI enzyme.
-

- 7. In the RFP Control tube: Add 1ul of EcoRI enzyme, and 1ul of PstI enzyme.
- 8. The total volume in each tube should be approximately 50ul. Mix well by pipetting slowly up and down 5x. Be gentle, and do not vortex. Spin the samples for 5 seconds in a microcentrifuge, or flick them to collect all of the mixture to the bottom of the tube.
- 9. Incubate the restriction digests at 37°C for 30 minutes, then 80°C for 20 minutes. We use a thermocycler, but a waterbath and an accurate thermometer works well also!
The digested DNA can be stored at 4°C for a few days. For longer storage, keep at -20°C.
Ligation
- 1. Clean the lab bench by wiping down with 70% ethanol and paper towels.
- 2. Thaw T4 DNA Ligase Reaction Buffer at room temperature. Keep the T4 DNA Ligase in the freezer until you're ready to use it.
- 3. Add 11μl of H2O to a 200μl PCR tube.
- 4. Add 2ul from each of the digest to the tube, Add 2μl of 10XT4 DNALigase Reaction buffer to the tube
- 5. Add 1ul of T4 DNA Ligase.
- 6. Mix by gently pipetting up and down 3x. Do not vortex; this inactivates the enzymes. Place tube in microcentrifuge for a quick 5 second spin or flick the tube to collect the mixture at the bottom. The total volume in each tube should now be 20 μl. Ensure the ligation is well-mixed by flick- ing the tube. You can spin the tube in a micro-
centrifuge for a few seconds to collect the liquid in the bottom of the tube again.
- 7. Incubate the reaction mix at room temperature for 10 min.
- 8. Incubate the reaction mix at 80C for 20 min. The 80C incubation deactivates the enzyme and improves transformation efficiency.
- 9. Store the ligation mix at -20C or proceed immediately to the transformation step.
Transformation
- 1. Clean the lab bench by wiping down with 70% ethanol and paper towels.
- 2. Keep all materials on ice unless otherwise specified! This will help make the cells more competent and easier to transform.
- 3. Keeping the tubes on ice for 10 minutes, pipet up and down gently 5x to re-suspend the cells.
- 4. Add 5ul of RFP control DNA (20ng/ul) into one the Transformation Control tube. Add 2ul of the ligated New Part product to its appropriate tube, and do the same for the Ligation Control. When adding the DNA pipet gently up and down 5x to mix the contents.
-

- 5. Incubate the DNA and cell mixtures on ice for 5 minutes.
-

- During the incubation, pre-heat the waterbath to 42° Celsius.
- 6. Place the tubes into the waterbath for 90 seconds. Place the tubes back on ice for 1 minute.
- 7. Add 200ul of SOC media to each tube. Pipet up and down gently to mix.
- 8. Label three agar plates containing the appropriate antibiotic with a corresponding tube.
- 9. Pipet 200ul of the Transformation Control onto the appropriately labelled plate. Carefully pour 3-6 glass beads into the plate. Spread evenly over the surface of the agar by gently shaking back and forth. Repeat this step for the other tubes.
- 10. Place the agar plates into the incubator with the agar side facing up, lid facing down. Incubate the agar plates at 37° Celsius for 12-14 hours. Alternately, incubate at room temperature for 24 hours.
-

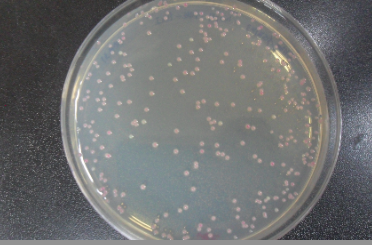
- 11. Check for red colonies the next day! Plates can be stored at 4° Celsius for a few days.
-
 "
"